Axenfeld-Rieger Syndrome, Type 1
A number sign (#) is used with this entry because of evidence that Axenfeld-Rieger syndrome type 1 (RIEG1) is caused by heterozygous mutation in the homeobox transcription factor gene PITX2 (601542) on chromosome 4q25.
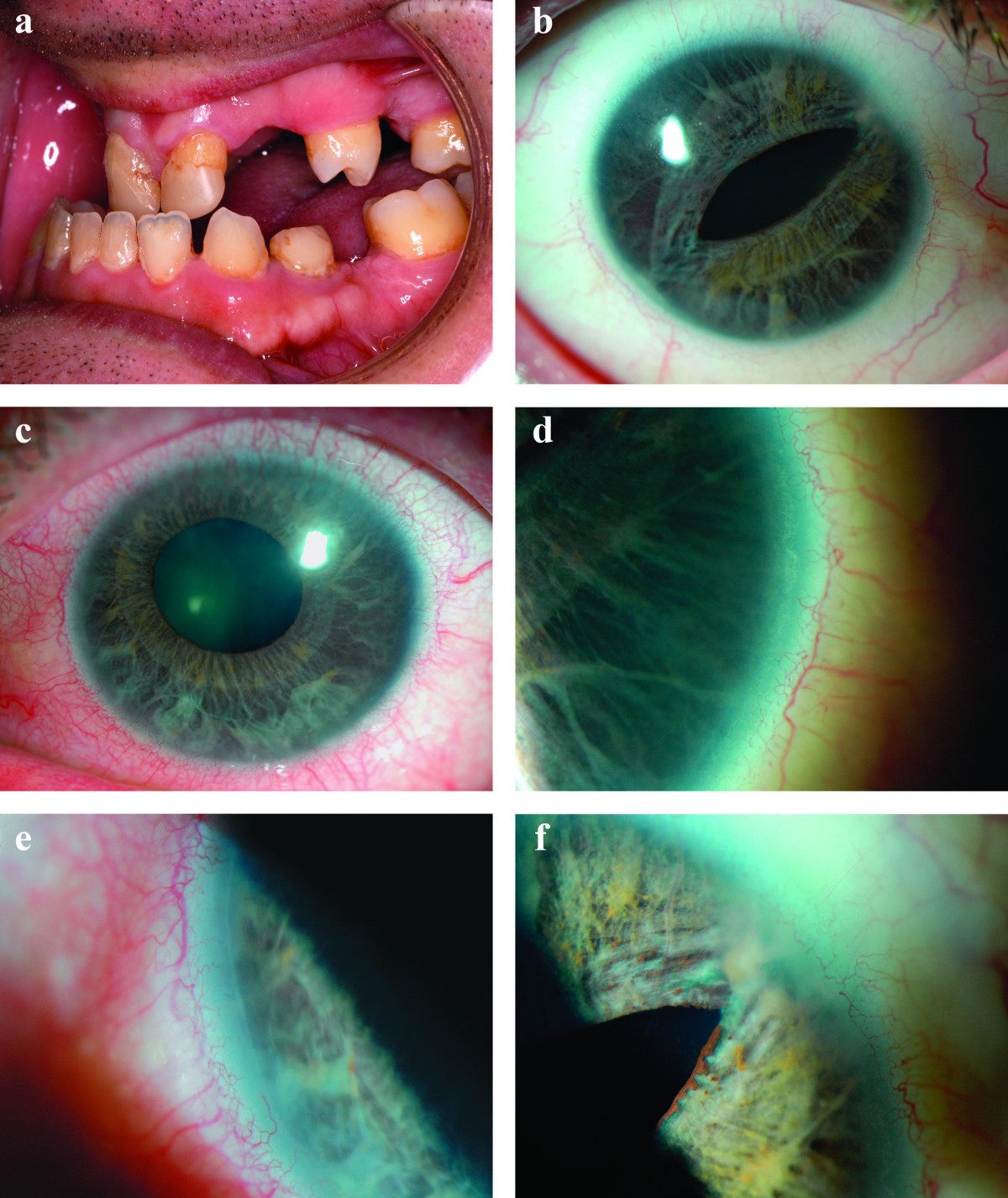
DescriptionAxenfeld-Rieger syndrome is an autosomal dominant disorder of morphogenesis that results in abnormal development of the anterior segment of the eye, and results in blindness from glaucoma in approximately 50% of affected individuals (Fitch and Kaback, 1978). Systemic anomalies are associated, including dental hypoplasia, failure of involution of periumbilical skin, and maxillary hypoplasia (Alkemade, 1969).
Genetic Heterogeneity of Axenfeld-Rieger Syndrome
Linkage studies indicate that a second type of Axenfeld-Rieger syndrome maps to chromosome 13q14 (RIEG2; 601499). A third form of Axenfeld-Rieger syndrome (RIEG3; 602482) is caused by mutation in the FOXC1 gene (601090) on chromosome 6p25.
See 109120 for a form of Axenfeld-Rieger syndrome associated with partially absent eye muscles, hydrocephalus, and skeletal abnormalities.
NomenclatureAlward (2000) reviewed the clinical features and molecular genetics of Axenfeld-Rieger syndrome and related disorders, noting that mutations in the 2 causative genes that had been identified, PITX2 and FOXC1, result in a wide variety of overlapping ocular phenotypes.
Sowden (2007) reviewed the molecular and developmental mechanisms of anterior segment dysgenesis and stated that the umbrella term Axenfeld-Rieger syndrome is best applied to the range of conditions with overlapping clinical features most commonly associated with PITX2 and FOXC1 mutations.
Clinical FeaturesHypodontia with malformation of the anterior chamber of the eye was recognized as a dominantly inherited disorder by Rieger (1935, 1941). The ocular features are microcornea with opacity, hypoplasia of the iris, and anterior synechiae. In 5 generations of a family, Busch et al. (1960) found myotonic dystrophy as a consistently associated feature. Others have not found myotonia. Pearce and Kerr (1965) studied a large kindred with many affected members and emphasized the variability in expression of the syndrome. A less well-known component of this syndrome is anal stenosis (Crawford, 1967; Brailey, 1890).
Alkemade (1969) amply confirmed autosomal dominant inheritance. He pointed out characteristic facies consisting of broad nasal root with telecanthus and maxillary hypoplasia with protruding lower lip. A mother and 2 of her 3 children had severe developmental anomalies of the iris, associated with maldevelopment of the ear and maxilla, umbilical hernia and anal stenosis. Glaucoma occurred in all 3 patients. It is doubtful that Axenfeld anomaly (defects limited to the peripheral anterior segment of the eye) should be considered a separate entity. It is one feature of Rieger syndrome. Feingold et al. (1969) observed 6 cases in 3 generations with male-to-male transmission.
De Hauwere et al. (1973) proposed that Rieger anomaly (peripheral abnormalities of the anterior segment with additional changes in the iris) with orbital hypertelorism and psychomotor retardation is a separate syndrome; see 109120.
Jorgenson et al. (1978) pointed out that 'failure of involution of the periumbilical skin' is a cardinal feature. Surgery for umbilical hernia had been performed in several. Friedman (1985) described the distinctive umbilical changes of Aarskog syndrome, Rieger syndrome, and Robinow syndrome. He quoted the famous monograph on the umbilicus by Cullen (1916) which has illustrations by Max Broedel. Toppare et al. (1995) in Turkey measured the length of the periumbilical skin in 304 newborn babies. On the cranial side of the base of the umbilical cord the skin measured 12.36 (SD 3.23) mm and the caudal umbilical skin measured 8.76 (SD 3.10) mm on the average. Toppare et al. (1995) suggested that if the cranial skin measurement is greater than 2 standard deviations beyond the mean, i.e., greater than 18.82 mm, Rieger syndrome should be considered.
Chisholm and Chudley (1983) reported a kindred with affected persons in 4 generations. Iridogoniodysgenesis was present in 10 persons, of whom 5 had established glaucoma. Somatic malformations were present in 5 persons in the third and fourth generations who did not have iridogoniodysgenesis. Nonocular features included characteristic facies (maxillary hypoplasia, short philtrum, and protruding lower lip of mild prognathism), dental anomalies (microdontia, hypodontia, and cone-shaped teeth), failure of involution of the umbilicus (often treated surgically in the neonatal period because of confusion with umbilical hernia), surgery for inguinal hernia in 8 persons, and hypospadias present in 4 males.
Brooks et al. (1989) described Rieger anomaly together with other anomalies and suggested that it represented a previously unreported syndrome. The patient, a sporadic case born to nonconsanguineous, young parents, had bilateral microcondyles and bilateral choanal atresia as well as anal atresia, scoliosis, kyphosis, and short stature. Dental findings included severe enamel hypoplasia, conical and misshapen teeth, hypodontia, and impactions. The maxilla and mandible were underdeveloped. Chromosome studies were not reported. Brooks et al. (1989) suggested that this 'new' syndrome be called the short-FRAME syndrome for short stature, facial anomalies, Rieger anomaly, midline anomalies, and enamel defects.
MappingShiang et al. (1987) studied DNA from a patient with a Rieger syndrome-like phenotype associated with a deletion at 4q23-q27. They found that probes for EGF (131530) and IL2 (147680) were deleted, whereas probes for ADH3 (ADH1C; 103710) were missing in a cell line that contained only the region of chromosome 4 from q25-qter. The patient had iris coloboma and delayed dentition in addition to other multiple anomalies. Since EGF and IL2 were deleted in the patient and FGFB (134920) may also be located in this region, they are possible candidate genes for Rieger syndrome.
Vaux et al. (1992) described a baby with features of Rieger syndrome associated with a de novo interstitial deletion of 4q that included band 4q26 and an adjoining Giemsa light band, either q25 or q27. Vaux et al. (1992) concluded that the Rieger syndrome locus is located in either 4q25 or 4q27 inasmuch as Motegi et al. (1988) found no signs of Rieger syndrome in a patient with deletion of band 4q26. Fryns and van den Berghe (1992) likewise found a deletion of the G-dark band 4q26 and of part of the G-light band 4q25 in a 4.5-year-old patient with Rieger syndrome and mental retardation. Using a group of highly polymorphic short tandem repeat polymorphisms (STRP), including a tetranucleotide repeat for EGF, Murray et al. (1992) identified linkage of Rieger syndrome to 4q markers. Tight linkage to EGF supported its role as a candidate gene, although a recombinant in an unaffected individual had been identified. It is possible that this unaffected person in fact carried the gene, which in him was nonpenetrant.
The possibility that autosomal dominant iris hypoplasia associated with early-onset glaucoma (137600) is allelic to Rieger syndrome was raised by the demonstration of Heon et al. (1995) of linkage to the same chromosomal region, 4q25. They studied a large family of Scandinavian descent who had a 5-generation history of iris hypoplasia. Iris hypoplasia was found in 15 individuals, 9 of whom had associated glaucoma. The highest observed lod score was 3.70 at theta = 0.0 for marker D4S1616.
In a mother with Rieger syndrome and polycystic ovaries (see 184700) and a son manifesting SHORT syndrome (269880), Karadeniz et al. (2004) identified a t(1;4)(q31.2;q25) translocation. The authors suggested that these syndromes may represent a single condition reflecting variable expression of the PITX2 gene.
Makita et al. (1995) described a boy with Rieger syndrome who had an apparently balanced reciprocal translocation between chromosomes 1 and 4. The clinical manifestations included irregular shaped pupils with a prominent Schwalbe line and an umbilical hernia. On cytogenetic studies, he was found to have a de novo reciprocal translocation 46,XY,t(1;4)(q23.1;q25), without visible deletion.
Walter et al. (1996) performed linkage analysis in the family originally described by Chisholm and Chudley (1983), in which iridogoniodysgenesis was combined with somatic abnormalities, and obtained a peak lod score of 7.827 (theta = 0.00) at D4S407 on chromosome 4q25.
Flomen et al. (1997) localized the proximal breakpoint of the constitutional deletion 4q in association with Rieger syndrome reported by Ligutic et al. (1981). They also described a new family with a de novo balanced reciprocal translocation t(4;12)(q25;q15) segregating with full Rieger syndrome in 2 generations. Using fluorescence in situ hybridization and P1 artificial chromosomes (PACs) as probes, Flomen et al. (1997) localized both the deletion and the translocation breakpoints between genetic markers that are known to be strongly linked to Rieger syndrome. They mapped both the proximal deletion breakpoint and the translocation breakpoint within a region between 2 groups of PACs bearing the markers for D4S2945 (on the centromeric side) and D4S193 and D4S2940 (on the telomeric side).
Genetic Heterogeneity
Nielsen and Tranebjaerg (1984) found partial monosomy of 21q22.2 in a case of Rieger syndrome. The patient had mental retardation, prominent occiput, enophthalmos, atresia of the right lacrimal duct, displaced anal opening, and supernumerary ribs. The mother had congenital stenosis of the lacrimal ducts. The proband had normal superoxide dismutase-1 (147450), confirming that the deletion was distal to 21q22.1. The malformation of the anterior chamber was manifest at birth by corneal clouding involving the stroma. The clouding gradually cleared over a few months except for a central opacity on the right associated with an anterior synechia. A sister of the maternal grandmother was said to have congenital corneal clouding. The authors reviewed the various chromosomal aberrations that have been found in association with an eye anomaly labeled Rieger syndrome; chromosomes 4, 6, 9, 13, and 18 have been implicated in addition to chromosome 21.
Legius et al. (1994) described a family with Rieger syndrome in 3 successive generations. Linkage to EGF and D4S193, localized in 4q25, was excluded. Legius et al. (1994) concluded that the disorder in this family was genetically distinct from typical Rieger syndrome. Although the patients had the eye malformation of that disorder with maxillary hypoplasia and hypertelorism, they did not show the dental or umbilical anomalies found in typical Rieger syndrome. The eye anomalies involving the anterior chamber of the eye varied from iris hypoplasia, iris strands, and uncomplicated glaucoma arising at adult age to congenital buphthalmos. Legius et al. (1994) pointed out that the chromosome anomalies other than those involving 4q point to different candidate regions for other forms of dominantly inherited Rieger eye malformation, with or without hypodontia.
Molecular GeneticsSemina et al. (1996) isolated the novel homeobox gene PITX2 (601542), which they designated RIEG, and identified 6 mutations in this gene (601542.0001-601542.0006) in individuals with Rieger syndrome.
PITX2 and DLX2 (126255) are transcription markers observed during early tooth development. Espinoza et al. (2002) demonstrated that PITX2 binds to bicoid and bicoid-like elements in the DLX2 promoter and activates this promoter 30-fold in Chinese hamster ovary cells. Mutations in PITX2 associated with Axenfeld-Rieger syndrome provided the first link of this homeodomain transcription factor to tooth development. One mutation produces Axenfeld-Rieger syndrome with iris hypoplasia but without tooth anomalies; this allele has a similar DNA binding specificity compared to wildtype PITX2 and transactivates the DLX2 promoter. In contrast, a different PITX2 mutation produces Rieger syndrome with the full spectrum of developmental anomalies, including tooth anomalies; this allele is unable to transactivate the DLX2 promoter. Since DLX2 expression is required for tooth and craniofacial development, the lack of tooth anomalies in the patient with iris hypoplasia may be due to the residual activity of this mutant in activating the DLX2 promoter. The authors proposed a molecular mechanism for tooth development involving DLX2 gene expression in Axenfeld-Rieger patients.
Lines et al. (2002) reviewed the molecular genetics of Axenfeld-Rieger malformations, including the roles of PITX2 and FOXC1 (601090) in human disease and mouse models.
HistorySchinzel (1987) gave an account of the life of Herwigh Rieger.