Cadasil
Summary
Clinical characteristics.
CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) is characterized by mid-adult onset of recurrent ischemic stroke, cognitive decline progressing to dementia, a history of migraine with aura, mood disturbance, apathy, and diffuse white matter lesions and subcortical infarcts on neuroimaging.
Diagnosis/testing.
The diagnosis of CADASIL is established in a proband either by identification of a heterozygous pathogenic variant in NOTCH3 by molecular genetic testing or, if molecular genetic testing is not definitive, by detection of characteristic findings by electron microscopy and immunohistochemistry of a skin biopsy.
Management.
Treatment of manifestations: There is no treatment of proven efficacy for CADASIL. Standard supportive treatment for stroke; the effect of thrombolytic therapy for the treatment of stroke remains unknown. Migraine should be treated symptomatically. Standard treatment for psychiatric disturbance. Supportive care (practical help, emotional support, and counseling) is appropriate for affected individuals and their families.
Prevention of primary manifestations: Antiplatelet therapy may be considered for prevention of stroke/TIA, but efficacy has not been proven. Anticoagulants should be avoided if possible. Control of vascular risk factors (hypertension, diabetes, hypercholesterolemia, smoking). Prophylactic treatment of migraines, depending on the frequency.
Surveillance: Routine evaluation by a neurologist with expertise in CADASIL; consultation with a neuropsychiatrist if symptoms of depression, apathy, or psychiatric manifestations; consultation with other medical specialists (e.g., rehabilitation physician, clinical geneticist, physical therapist, and psychologist) as needed.
Agents to avoid: Thrombolytic therapy (intravenous thrombolysis) and oral anticoagulants probably increase the risk of intracerebral hemorrhage in individuals with CADASIL. These agents should therefore only be used carefully and on a case-by-case basis. Smoking increases the risk of stroke.
Pregnancy management: There may be an increased risk for neurologic events in pregnancy during and shortly after delivery (puerperium); transient neurologic events (migraine with aura) have most commonly been reported.
Genetic counseling.
CADASIL is inherited in an autosomal dominant manner. Most affected individuals have an affected parent; de novo pathogenic variants appear to be rare. Each child of an affected person is at a 50% risk of inheriting the pathogenic variant and developing signs of the disease. Prenatal testing for a pregnancy at increased risk and preimplantation genetic testing are possible if the pathogenic variant in the family is known.
Diagnosis
There are no generally accepted diagnostic criteria for CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy). A CADASIL diagnostic screening tool has been proposed by Pescini et al [2012], Mizuta et al [2017], and Bersano et al [2018].
Suggestive Findings
CADASIL should be suspected in individuals with unexplained white matter hyperintensities and a family history of stroke and/or vascular dementia; however, lack of an apparent family history of CADASIL does not preclude the diagnosis (see Family history). The following clinical signs and neuroimaging findings can be observed in CADASIL.
Clinical signs
- Transient ischemic attacks and ischemic stroke
- Cognitive impairment, manifesting initially with executive dysfunction, with a concurrent stepwise deterioration due to recurrent strokes to vascular dementia
- Migraine with aura, with a mean age of onset of 30 years
- Psychiatric disturbances, most frequently mood disturbances and apathy
Brain imaging
- Symmetric and progressive white matter hyperintensities, often involving the anterior temporal lobes and external capsules
- Lacunes of presumed vascular origin
- Recent subcortical infarcts
- Dilated perivascular spaces, sometimes referred to as subcortical lacunar lesions
- Brain atrophy
- Cerebral microbleeds
Family history consistent with autosomal dominant inheritance is suggestive. Note, however, that lack of an apparent family history of CADASIL does not preclude the diagnosis, as affected family members may have been misdiagnosed [Razvi et al 2005a] and de novo cases have been described [Joutel et al 2000, Coto et al 2006].
Establishing the Diagnosis
The diagnosis of CADASIL is established in a proband either by identification of a heterozygous pathogenic variant in NOTCH3 by molecular genetic testing (see Table 1) or, if molecular genetic testing is not definitive, by detection of characteristic findings by electron microscopy and immunohistochemistry of a skin biopsy.
Molecular genetic testing approaches can include a combination of gene-targeted testing (single-gene testing, multigene panel) and comprehensive genomic testing (exome sequencing, exome array, genome sequencing) depending on the phenotype.
Gene-targeted testing requires that the clinician determine which gene(s) are likely involved, whereas genomic testing does not. Because the phenotype of CADASIL is broad, individuals with the distinctive findings described in Suggestive Findings are likely to be diagnosed using gene-targeted testing (see Option 1), whereas those with a phenotype indistinguishable from many other inherited disorders with recurrent stroke and/or dementia are more likely to be diagnosed using genomic testing (see Option 2).
Option 1
When the phenotypic and laboratory findings suggest the diagnosis of CADASIL, molecular genetic testing approaches can include single-gene testing or use of a multigene panel:
- Single-gene testing. Sequence analysis of NOTCH3 (to include, at a minimum, exons 2-24) detects small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected.Perform sequence analysis first. If a biallelic pathogenic variant is identified, consider gene-targeted deletion/duplication analysis to determine if an unidentified exon deletion or duplication is present.Note: Biallelic NOTCH3 pathogenic variants have been described in individuals with CADASIL (see Genotype-Phenotype Correlations).
- A multigene panel that includes NOTCH3 and other genes of interest (see Differential Diagnosis) is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.For an introduction to multigene panels click here. More detailed information for clinicians ordering genetic tests can be found here.
Option 2
When the phenotype is indistinguishable from many other inherited disorders characterized by stroke and/or dementia, comprehensive genomic testing (which does not require the clinician to determine which gene[s] are likely involved) is the best option. Exome sequencing is most commonly used; genome sequencing is also possible.
For an introduction to comprehensive genomic testing click here. More detailed information for clinicians ordering genomic testing can be found here.
Table 1.
Molecular Genetic Testing Used in CADASIL
| Gene 1 | Test Method | Proportion of Probands with a Pathogenic Variant 2 Detectable by This Method |
|---|---|---|
| NOTCH3 | Sequence analysis 3 | Estimated >95% 4 |
| Gene-targeted deletion/duplication analysis 5 | Unknown 6 |
- 1.
See Table A. Genes and Databases for chromosome locus and protein.
- 2.
See Molecular Genetics for information on allelic variants detected in this gene.
- 3.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Pathogenic variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 4.
When all exons coding for epidermal growth factor-like repeats (see Molecular Genetics) are sequenced (exons 2-24) and stringent inclusion criteria are applied [Markus et al 2002, Peters et al 2005a]
- 5.
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications.
- 6.
Rutten et al [2013]
Skin biopsy. The diagnosis can be confirmed by ultrastructural analysis of small arterioles obtained, for example, by skin biopsy [Goebel et al 1997, Ruchoux & Maurage 1997]. Electron microscopy shows characteristic granular osmophilic material (GOM) within the vascular media close to vascular smooth muscle cells. The detection of GOM is considered pathognomonic for CADASIL, but the reported sensitivity is variable [Tikka et al 2009, Morroni et al 2013].
NOTCH3 immunostaining of a skin biopsy shows a positive granular NOTCH3 staining of the vessel wall [Joutel et al 2001, Oberstein 2003].
The combined analysis by electron microscopy and immunohistochemistry, when interpreted by an experienced (neuro)pathologist, usually allows for a conclusive CADASIL diagnosis [Rutten et al 2014].
Clinical Characteristics
Clinical Description
CADASIL is a disease of the small to medium-sized arteries, mainly affecting the brain. The presenting symptoms, age at onset, and disease progression in CADASIL are variable, both between and within families. The disease is characterized by five main symptoms: transient ischemic attacks and recurrent ischemic strokes; cognitive decline; migraine with aura; mood disturbance; and apathy.
Subcortical ischemic events. Transient ischemic attacks (TIAs) and stroke, the most frequent presentation, are found in approximately 85% of symptomatic individuals [Dichgans et al 1998]. Mean age at onset for ischemic episodes is 47 years (age range 20-70 years) [Opherk et al 2004].
Ischemic episodes typically present as a classic lacunar syndrome (pure motor stroke, ataxic hemiparesis/dysarthria-clumsy hand syndrome, pure sensory stroke, sensorimotor stroke), but other lacunar syndromes (brain stem or hemispheric) are also observed [Adib-Samii et al 2010]. Ischemic episodes are often recurrent, leading to severe disability with gait disturbance, urinary incontinence, and pseudobulbar palsy.
Strokes involving the territory of a large artery have occasionally been reported. However, whether these are coincidental observations, or whether (certain sub-populations of) individuals with CADASIL are at increased risk for large vessel stroke, is unclear [Choi et al 2013, Yin et al 2015, Kang & Kim 2015].
Cognitive deficits and dementia. Cognitive decline may start as early as age 35 years. Up to 75% of affected individuals develop dementia, often accompanied by apathy [Dichgans 2009, Reyes et al 2009].
The pattern of cognitive dysfunction is initially characterized by deficits in executive function (timed measures and measures of error monitoring), verbal fluency, and memory with benefit from clues [Peters et al 2005b]. Cognitive dysfunction is accompanied by a narrowing of the field of interest. In most cases, cognitive decline is slowly progressive, with some preservation of recognition and semantic memory, with additional stepwise deterioration. Visuospatial abilities and reasoning decline, especially after age 60. Cognitive decline becomes more apparent with aging and disease progression, ultimately leading to significant alterations in all cognitive domains at an older age [Buffon et al 2006]. Amberla et al [2004] observed deterioration of working memory and executive function in individuals with NOTCH3 pathogenic variants in the pre-stroke phase and inferred that cognitive decline may start insidiously before the onset of symptomatic ischemic episodes.
Migraine, when present, can be the first symptom of CADASIL. Migraine occurs in 30%-75% of individuals with CADASIL, with the first attack occurring at a mean age of 26-29 years [Adib-Samii et al 2010, Tan & Markus 2016, Guey et al 2016]. Eighty to ninety percent of those with migraine have migraine with aura [Guey et al 2016, Tan & Markus 2016]. Migraine auras are sometimes confused with transient ischemic symptoms, since aura may include focal neurologic deficits [Di Donato et al 2017]. Sixty percent of those with migraine with aura have experienced an atypical migraine attack: prolonged, basilar or hemiplegic aura, confusion, fever, or coma [Guey et al 2016].
Psychiatric disorders. In most CADASIL cohorts, psychiatric disturbances are described in about one third of individuals [Adib-Samii et al 2010]. The reported prevalence of psychiatric disturbances is variable: a small study in 23 Italian patients recorded a lifetime risk for depression of 74% and a lifetime risk for a manic episode of 26% [Valenti et al 2011], whereas in a Chinese cohort, psychiatric manifestations were recorded in only 7% of affected individuals [Wang et al 2011]. Apathy has been described in 40% of individuals [Reyes et al 2009]. The psychiatric manifestations vary from personality changes to severe depression. The pathogenesis of psychiatric disturbances in CADASIL is incompletely understood. Psychiatric problems as a presenting symptom of CADASIL have been described [Leyhe et al 2005, Nakamura et al 2005, Park et al 2014].
Reversible acute encephalopathy. Acute encephalopathy has been described in some individuals, with confusion, headache, pyrexia, seizures, and coma, sometimes leading to death [Adib-Samii et al 2010, Ragno et al 2013, Tan & Markus 2016]. Migraine with aura (especially confusional aura) is associated with increased risk of acute encephalopathy, suggesting that they may share pathophysiologic mechanisms [Tan & Markus 2016].
Epilepsy. Epilepsy occurs in 10% of individuals with CADASIL and presents in middle age, usually secondary to stroke [Haan et al 2007]. In a pooled analysis of previous published cases, three of 105 individuals with CADASIL had a seizure as the initial presenting symptom [Desmond et al 1999].
Pregnancy. It has been suggested that the risk for migraine with aura is increased during pregnancy, but especially during puerperium (the period between childbirth and the return of the uterus to its normal size) [Roine et al 2005]. Another study found no association between pregnancy and risk for neurologic events or problems during pregnancy [Donnini et al 2017]. See Pregnancy Management.
Other findings
- Cardiac. Controversy exists as to whether CADASIL is associated with cardiac involvement. In a study from The Netherlands, nearly 25% of individuals with CADASIL had a history of acute myocardial infarction (MI) and/or current pathologic Q-waves on electrocardiogram (ECG) [Lesnik Oberstein et al 2003]. This percentage was significantly higher than in controls without a heterozygous NOTCH3 pathogenic variant. However, another study of 23 individuals with CADASIL found no signs of previous MI on ECG [Cumurciuc et al 2006b]. Two studies have suggested an increased risk for arrhythmias, based on increased QT variability on ECG recording [Rufa et al 2007, Piccirillo et al 2008].
- Nerve. Nerve biopsies may demonstrate signs of axonal damage, demyelination, and ultrastructural changes of the endoneurial blood vessels [Schröder et al 2005, Sicurelli et al 2005, Lackovic et al 2012]. Punch skin biopsies from individuals with CADASIL showed cutaneous somatic and autonomic nerve involvement [Nolano et al 2016]. Clinically, however, there is no clear evidence that peripheral neuropathy is part of the CADASIL clinical spectrum [Kang et al 2009].
- Ocular. Subclinical retinal lesions are reported [Cumurciuc et al 2004, Haritoglou et al 2004]. Fundoscopy may reveal clinically silent retinal vascular abnormalities [Pretegiani et al 2013]. Optical coherence tomography imaging techniques may show reduced subfoveal choroidal thickness, retinal arterial luminal narrowing, retinal venous luminal enlargement, and reduced vessel density of the deep retinal plexus [Alten et al 2014, Fang et al 2017, Nelis et al 2018].
- Renal. NOTCH3 accumulation and granular osmophilic material (GOM) are also detected in renal arteries, and stenosis of renal arteries has been described [Ragno et al 2012, Lorenzi et al 2017]. Although no large-scale studies have been published regarding kidney function in individuals with CADASIL, to date there is no evidence that kidney function is affected [Bergmann et al 1996].
Long-term prognosis and causes of death. Onset of symptoms and overall survival may vary based on the type of pathogenic variant and its location within NOTCH3 (see Genotype-Phenotype Correlations).
Data on the long-term prognosis in CADASIL come from a large study of 411 individuals [Opherk et al 2004], which found that the median age at onset of inability to walk without assistance was approximately 60 years and the median age at which individuals became bedridden was 64 years. The median age at death was 68 years with a more rapid disease progression in men than in women. Pneumonia was the most frequent cause of death, followed by sudden unexpected death and asphyxia. In the final stages of disease, 78% of individuals were completely dependent and 63% were confined to bed.
The median survival time of men was significantly shorter than expected from German life tables, whereas the median survival time of women was not significantly reduced. The reason for this difference is not known; possible explanations include sex hormones, sex differences in risk factor control, medical management, social support, and socioeconomic factors.
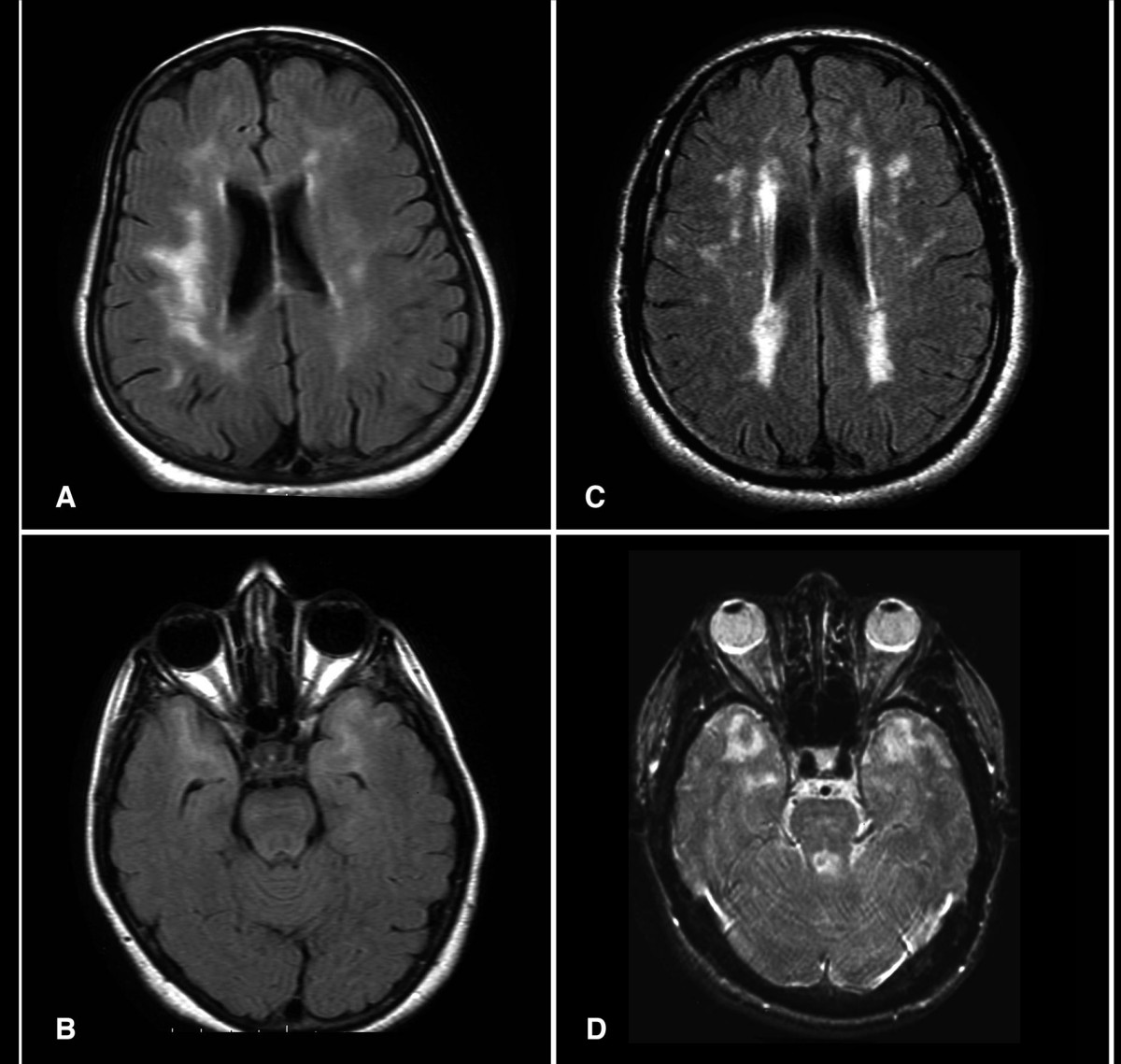
Brain imaging. Imaging abnormalities in CADASIL evolve as the disease progresses [van den Boom et al 2003, Singhal et al 2005, Liem et al 2008a]:
- In individuals age 20-30 years, distinctive white matter hyperintensities often first appear in the anterior temporal lobes, when the rest of the white matter, except for periventricular caps, appears unaffected [Oberstein 2003, van den Boom et al 2003].
- In the course of the disease, the load of white matter hyperintensity lesions increases, eventually coalescing to the point where, in some elderly individuals, normal-appearing white matter is barely distinguishable [Chabriat et al 1998].
- White matter hyperintensities in the temporal lobe and external capsule are characteristic for CADASIL but not always present [O’Sullivan et al 2001, Markus et al 2002]. Involvement of the anterior temporal lobe is highly suggestive for CADASIL; this finding, however, is not sensitive or specific for the diagnosis of CADASIL [Sureka &Jakkani 2012].
- In symptomatic individuals, white matter hyperintensities are symmetrically distributed and located in the periventricular and deep white matter. Within the white matter, the frontal lobe is the site with the highest lesion load, followed by the temporal and parietal lobes [Auer et al 2001, O'Sullivan et al 2001].
- The majority of lacunes develop at the edge of white matter hyperintensities and proximal to white matter hyperintensities along the course of perforating vessels supplying the respective brain region [Duering et al 2013].
- Brain atrophy appears to result from accumulation of lacunes and widespread microstructural alterations within the brain [Jouvent et al 2007].
- Lacunes and brain atrophy are strongly correlated with clinical severity and clinical worsening in individuals with CADASIL [Ling et al 2018, Jouvent et al 2016].
- Dilated perivascular spaces are found in approximately 70%-80% of affected individuals [van Den Boom et al 2002, Cumurciuc et al 2006a, Yao et al 2014].
- Cerebral microbleeds (CMB) are reported in approximately one third of affected individuals [Puy et al 2017, Nannucci et al 2018]. Cerebral microbleeds do not have a clear predominant location in individuals with CADASIL. In a study of 125 affected individuals, cerebral microbleeds were present in 34%. Of these, 29% had CMB in the deep subcortical region (most frequently in the thalamus), 22% in the lobar region (especially the temporal lobe), and 18% in the infratentorial region [Nannucci et al 2018].
Other imaging studies. Positron emission tomography, transcranial Doppler sonography, and perfusion MRI may show decreased cerebral blood flow, decreased cerebral blood volume, impaired cerebral metabolism, decreased vasoreactivity, and altered neurovascular coupling [Tatsch et al 2003, Tuominen et al 2004, Huneau et al 2018, Moreton et al 2018].
Pathophysiology. Cerebral blood supply in individuals with CADASIL is reduced below demand, as demonstrated by an increased oxygen extraction rate in asymptomatic and demented individuals with CADASIL. Cerebral blood flow, cerebral blood volume, and cerebral glucose utilization are significantly reduced [Bruening et al 2001, Tuominen et al 2004]. In addition, cerebral vasoreactivity is impaired [Pfefferkorn et al 2001], consistent with the observed degeneration of vascular smooth muscle cells in small arteries and arterioles [Kalimo et al 2002]. Increased fragility of cerebral microvessels is suggested by a high frequency of cerebral microbleeds at autopsy and on gradient echo MRI [Lesnik Oberstein et al 2001, Dichgans et al 2002].
Genotype-Phenotype Correlations
Smaller studies have described genotype-phenotype correlations for specific pathogenic variants [Lesnik Oberstein et al 2001, Arboleda-Velasquez et al 2002, Opherk et al 2004, Bianchi et al 2010]; however, none of these associations have been firmly established.
In general, affected individuals with cysteine-altering pathogenic variants in epidermal growth-factor like repeat (EGFr) domains 1-6 of NOTCH3 have a 12-year earlier onset of stroke, lower survival, and increased white matter hyperintensity volume, consistent with the more severe classic CADASIL presentation, compared to those with a cysteine-altering pathogenic variant in EGFr domains 7-34. The mean survival time was 68.5 and 76.9 years, respectively [Rutten et al 2019].
There is conflicting evidence about the effect of pathogenic variants in the ligand-binding domain of NOTCH3 (EGFr domains 10 and 11). Both a more severe and a milder phenotype have been described in small series [Arboleda-Velasquez et al 2002, Monet-Leprêtre et al 2009, Rutten et al 2019] (see also Penetrance).
Biallelic pathogenic variants in NOTCH3 have been described in individuals with CADASIL [Tuominen et al 2001, Liem et al 2008b, Ragno et al 2013, Soong et al 2013, Vinciguerra et al 2014, Abou Al-Shaar et al 2016]. The phenotype of individuals with biallelic NOTCH3 pathogenic variants falls within the CADASIL spectrum.
Penetrance
Pathogenic variants in EGFr domains 1-6 appear to be fully penetrant and are usually associated with the classical CADASIL phenotype. However, there is variability in disease severity.
Pathogenic variants in EGFr domains 7-34 have a much higher population frequency (~1:300) [Rutten et al 2016a, Rutten et al 2019] and therefore likely predispose to a milder small-vessel disease and may even be non-penetrant.
Nomenclature
Previous descriptions of families with "hereditary multi-infarct dementia," "chronic familial vascular encephalopathy," and "familial subcortical dementia" represent early reports of CADASIL.
Prevalence
While most published information on individuals with CADASIL originates from Europe, CADASIL has been observed on all continents. Multiple small and national European registries have estimated the minimum prevalence at between two and four per 100,000 [Kalimo et al 2002, Razvi et al 2005b, Narayan et al 2012, Bianchi et al 2015].
Recently, it was found that the frequency of NOTCH3 cysteine-altering pathogenic variants is 1:300 in the general population worldwide (gnomAD), with the highest frequency in people who are from Asian descent (1:100) [Rutten et al 2016a, Rutten et al 2019]. This is approximately 100-fold higher than current estimates of the minimum prevalence of CADASIL, suggesting that CADASIL is much more prevalent than previously suspected and that the NOTCH3 clinical spectrum must be considerably broader, ranging from classic CADASIL to a much milder small-vessel disease, or possibly even non-penetrance.
Differential Diagnosis
The differential diagnosis of CADASIL includes sporadic/multifactorial disorders and inherited disorders.
Sporadic/Multifactorial Disorders
The clinical characteristics and MRI abnormalities in these conditions may resemble those of CADASIL. The presence of temporopolar MRI lesions, the absence of optic nerve and spinal cord involvement, the absence of oligoclonal bands in the cerebrospinal fluid, and the absence of hypertension are critical in this regard [Dichgans et al 1999] (see Table 2).
Table 2.
Clinical Signs and MRI Abnormalities of Sporadic/Multifactorial Disorders in the Differential Diagnosis of CADASIL
| Disorder | Distinguishing Clinical Characteristics | Distinguishing MRI Abnormalities | Temporopolar MRI Lesions | Optic Nerve & Spinal Cord Involvement | Oligoclonal Bands in CSF | Hypertension |
|---|---|---|---|---|---|---|
| Multiple sclerosis 1 |
|
| Common | Typical | Yes | Not associated |
| Sporadic small vessel disease incl Binswanger's disease |
|
| Rare | No | No | Associated |
| Primary angiitis of the nervous system 2 |
|
| Uncommon | Involvement of:
| Occasionally | Not associated |
AD = autosomal dominant; AR = autosomal recessive; CSF = cerebrospinal fluid; WMH = white matter hyperintensities
- 1.
Joshi et al [2017]
- 2.
Williamson et al [1999]
Inherited Disorders
Several inherited disorders are associated with acute ischemic events (or stroke-like episodes in the case of MELAS) and cerebral white matter hyperintensities on MRI. These disorders can be distinguished from CADASIL by the associated clinical signs, MRI, mode of inheritance, and appropriate laboratory investigations (particularly, molecular genetic testing) (see Table 3).
Table 3.
Clinical Signs and MRI Abnormalities of Inherited Disorders to Consider in the Differential Diagnosis of CADASIL
| Disorder | Gene(s) | MOI | Distinguishing Clinical Signs | Distinguishing MRI Abnormalities |
|---|---|---|---|---|
| Fabry disease | GLA | XL | Classic Fabry disease phenotype:
|
|
| CARASIL 1 | HTRA1 | AR |
|
|
| HTRA1 cerebral small vessel disease (CADASIL type 2) | AD |
| Spinal spondylosis | |
| MELAS 2 | MT-TL1 MT-ND5 3 | Mat |
|
|
| CARASAL 4 | CTSA | AD |
| Intracerebral hemorrhages |
| COL4A1- & COL4A2- related small vessel disease (see COL4A1-Related Disorders & OMIM PS175780) 5 | COL4A1 COL4A2 | AD |
|
|
AD = autosomal dominant; AR = autosomal recessive; Mat = maternal; MOI = mode of inheritance; WMH = white matter hyperintensities; XL = X-linked
- 1.
CARASIL = cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy
- 2.
MELAS = mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes
- 3.
Pathogenic variants known to cause MELAS have been identified in other mtDNA tRNA genes including MT-TC, MT-TK, MT-TV, MT-TF, MT-TQ, MT-TS1, MT-TS2, and MT-TW, and in the protein-encoding genes MT-CO1, MT-CO2, MT-CO3, MT-CYB, MT-ND1, MT-ND3, and MT-ND6.
- 4.
CARASAL = cathepsin A–related arteriopathy with strokes and leukoencephalopathy [Bugiani et al 2016]
- 5.
Meuwissen et al [2015]
Management
Evaluations Following Initial Diagnosis
To establish the extent of disease and needs in an individual diagnosed with CADASIL, the following evaluations are recommended, if they have not already been completed.
Table 4.
Recommended Evaluations Following Initial Diagnosis in Individuals with CADASIL
| System/Concern | Evaluation | Comment |
|---|---|---|
| Neurologic | Complete neurologic evaluation | |
| Standard brain MRI incl FLAIR sequence & T2-weighted gradient echo imaging | To determine the extent of disease & to have a baseline measure for follow up | |
| Neurodevelopmental | Psychometrics | W/particular attention to executive function |
| Psychiatric/ Behavioral | Consider referral to a psychiatrist. | For treatment of mood disorders & evaluation of apathy |
| Miscellaneous/ Other | Consultation w/a clinical geneticist &/or genetic counselor | To include genetic counseling |
| Family support/resources | Assess use of community or online resources. | |
| Social work involvement for support | ||
| Home nursing referral, if needed |
Treatment of Manifestations
Discussion of medical management options for individuals with CADASIL have been published [del Río-Espínola et al 2009, Di Donato et al 2017].
Table 5.
Treatment of Manifestations in Individuals with CADASIL
| Manifestation/ Situation | Treatment | Considerations/Other |
|---|---|---|
| Acute stroke/ TIA | Standard supportive treatment following the guidelines of stroke medicine | The effect of thrombolytic therapy (intravenous thrombolysis) is unknown; there is no evidence or rationale for thrombolysis in those w/CADASIL, and the risk of intracerebral hemorrhage is probably increased; see Agents/Circumstances to Avoid. |
| Migraine | Symptomatic treatment, which may include triptans & ergot derivatives | There is NO evidence that triptans or ergot derivatives are contraindicated due to their vasoconstrictive mechanism of action. 1 |
| Psychiatric disturbance | Standard treatment 2 | Psychometric & psychiatric evaluation can be used to evaluate whether apathy (if present) is a symptom of depression or of cognitive dysfunction. |
TIA = transient ischemic attack
- 1.
Triptans appear to have the same treatment responses and frequency of serious side effects in individuals with CADASIL as in the general migraine population [Tan & Markus 2016].
- 2.
No studies have been performed in individuals with CADASIL to determine the effect of the treatment of psychiatric disturbances (e.g. depression) with psychiatric drugs.
Prevention of Primary Manifestations
No treatment is of proven efficacy in preventing stroke, vascular dementia, or CADASIL disease progression.
Table 6.
Prevention of Primary Manifestations in Individuals with CADASIL
| Manifestation/ Situation | Prevention | Considerations/Other |
|---|---|---|
| Stroke/TIA | Consider antiplatelet therapy. 1 | The effect of antiplatelet therapy in individuals w/CADASIL is unknown and there is no evidence or rationale for its use in treatment of CADASIL – though also no evidence that it is contraindicated. |
| Control of vascular risk factors | Incl hypertension, diabetes, hypercholesterolemia, & smoking | |
| Migraine | Consider prophylactic therapy. | Depending on migraine frequency |
- 1.
Such as aspirin and clopidogrel
Surveillance
No standard international surveillance guidelines for CADASIL exist. Several countries have developed CADASIL guidelines, such as the medical guideline for CADASIL published (in French) by the French Health Authority (HAS).
The interval at which individuals with CADASIL should be seen for follow up depends on the severity and type of symptoms and the needs of patients and their care givers.
- Follow up by a neurologist with expertise in CADASIL is recommended from the time of diagnosis onward.
- A consultation with a neuropsychiatrist is recommended when there are symptoms of depression, apathy, or other psychiatric manifestations.
- Consultation of other medical specialists (e.g., rehabilitation physician, clinical geneticist, physical therapist, and psychologist) is as required.
Agents/Circumstances to Avoid
The treatment effect of thrombolytic therapy (intravenous thrombolysis) is unknown in individuals with CADASIL. Studies performed in non-CADASIL populations show that increasing cerebral microbleed burden and increasing white matter hyperintensity lesion load is associated with an increased risk of intracerebral hemorrhage after thrombolytic therapy [Charidimou et al 2016, Charidimou et al 2017b]; therefore, individuals with CADASIL may be at increased risk for intracerebral hemorrhage. In case of a thromboembolic large vessel stroke (i.e., unrelated to CADASIL), the benefit of thrombolysis outweighs the potential risk of intracerebral hemorrhage.
Oral anticoagulants could lead to an increased risk of intracerebral hemorrhage in individuals with CADASIL due to the presence of microbleeds [Charidimou et al 2017a]; the prescription of anticoagulants should therefore be carefully weighed. In case of a clear indication for anticoagulant therapy, such as atrial fibrillation or deep venous thrombosis, the benefit of the treatment likely outweighs the potential risk of intracerebral hemorrhage.
Smoking increases the risk of stroke in individuals with CADASIL and should be avoided [Singhal et al 2004].
Evaluation of Relatives at Risk
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Pregnancy Management
Fetuses affected with CADASIL are not at an increased risk for intrauterine complications or complications during/after delivery [Donnini et al 2017].
In a retrospective study, women with CADASIL were at increased risk for neurologic events in pregnancy during and shortly after delivery (puerperium) [Roine et al 2005]. However, another retrospective study of 50 affected women and prospectively collected data of six women showed no association between CADASIL and risk for neurologic events or problems during pregnancy [Donnini et al 2017]. In the authorsʹ experience, most women with CADASIL have an uncomplicated pregnancy and delivery, but transient neurologic events are sometimes reported (mostly consistent with migraine aura) [Lesnik Oberstein, unpublished observation based on clinical practice].
Therapies Under Investigation
Case reports and small-scale observational studies have suggested a beneficial effect of acetazolamide on migraine [Weller et al 1998, Forteza et al 2001, Donnini et al 2012]. Acetazolamide has also been shown to have a beneficial effect on cerebral perfusion [Chabriat et al 2000, Huang et al 2010, Park et al 2011, Fujiwara et al 2012]. Acetazolamide is not given to individuals with CADASIL on a routine basis, as no large studies have been performed.
Several therapeutic approaches are in pre-clinical development: testing in cells and mouse models including immunotherapy [Machuca-Parra et al 2017, Ghezali et al 2018], antisense mediated NOTCH3 exon skipping [Rutten et al 2016b], and treatment with stem cell factor and granulocyte colony-stimulating factor [Liu et al 2015].
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for access to information on clinical studies for a wide range of diseases and conditions.
Other
Cross-sectional and longitudinal studies suggest that disease progression is faster in individuals with CADASIL who have increased blood pressure [Peters et al 2004, Holtmannspötter et al 2005, Peters et al 2006, Ling et al 2017]. However, no controlled data regarding the effect of antihypertensive treatment on disease progression are available.