Pitt-Hopkins Syndrome
Summary
Clinical characteristics.
Pitt-Hopkins syndrome (PTHS) is characterized by significant developmental delays with moderate-to-severe intellectual disability and behavioral differences, characteristic facial features, and episodic hyperventilation and/or breath-holding while awake. Speech is significantly delayed and most individuals are nonverbal with receptive language often stronger than expressive language. Other common findings are autism spectrum disorder symptoms, sleep disturbance, stereotypic hand movements, seizures, constipation, and severe myopia.
Diagnosis/testing.
The diagnosis is suspected on clinical findings and confirmed by identification on molecular genetic testing of a heterozygous pathogenic variant in TCF4 or a deletion of the chromosome region in which TCF4 is located (18q21.2).
Management.
Treatment of manifestations: Developmental services for infants (physical, occupational, and speech therapies); individualized education plan for older children with strong consideration for early training in alternative means of communication; behavioral management strategies; possible treatment of abnormal respiratory pattern. Routine management of seizures, myopia, constipation, scoliosis, and ankle instability.
Surveillance: Ongoing developmental assessments to tailor educational services to individual needs; regular follow up with an ophthalmologist to monitor for high myopia and strabismus; periodic reevaluation with a clinical genetics professional regarding current information and recommendations.
Genetic counseling.
PTHS is caused by haploinsufficiency of TCF4 resulting from either a pathogenic variant in TCF4 or a deletion of the chromosome region in which TCF4 is located (18q21.2). Most affected individuals have been simplex cases (i.e., a single occurrence in a family) resulting from a de novo pathogenic variant or deletion. The risk to sibs of a proband is low, but higher than that of the general population because of the possibility of parental germline mosaicism. Once the PTHS-related genetic alteration has been identified in an affected family member, prenatal testing for a pregnancy at increased risk and preimplantation genetic diagnosis are possible.
Diagnosis
Pitt-Hopkins syndrome (PTHS) is a rare genetic disorder characterized by developmental delay, intellectual disability, behavioral differences, distinctive facial features, and unusual breathing patterns.
Suggestive Findings
PTHS should be suspected in individuals with developmental delay, moderate-to-severe intellectual disability, behavioral differences, characteristic facial features, and episodic hyperventilation and/or breath-holding while awake.
Developmental delay / intellectual disability / behavioral differences
- Motor milestones are delayed; often with hypotonia.
- Speech is severely limited to absent, with a history of regression in verbal abilities in some.
- Intellectual disability is typically moderate to severe.
- Autism spectrum disorder (ASD) symptoms are also common, though social engagement is generally present.
- Other behavioral characteristics suggestive of PTHS include the following:
- Love of music
- Frequent smiling
- Stereotypic hand movements
- Hand flapping
- Spontaneous laughter
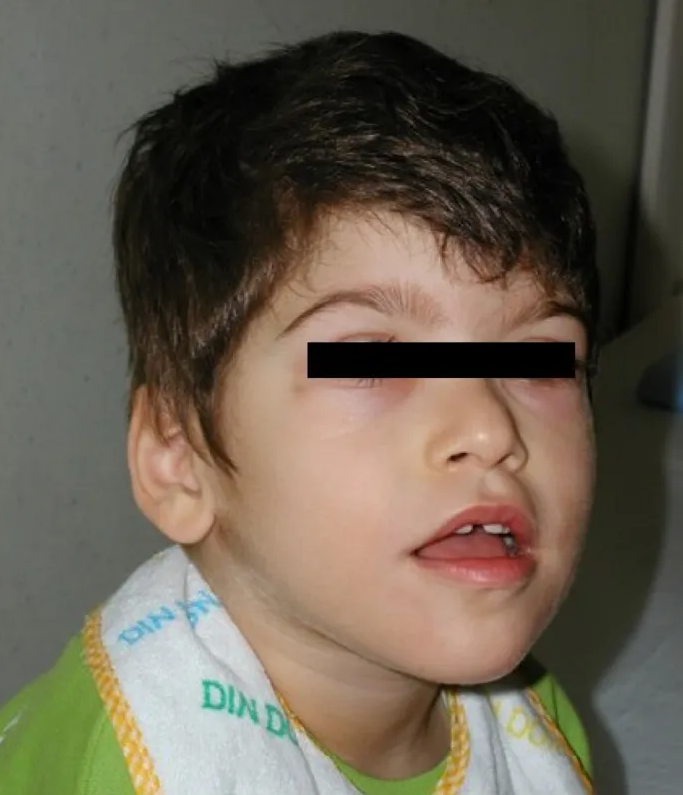
Characteristic facial features that become more apparent with age. The craniofacial features are an important aspect for the diagnosis of PTHS, but may be less obvious in infancy. In many individuals, the prominence of the nose and lower face may be the earliest clue to PTHS in an infant with developmental concerns (see Figures 1, 2, 3, 4, 5).
 syndrome.">
syndrome.">Figure 1.
Newborn male with Pitt-Hopkins syndrome. Note high nasal root with prominent nasal bridge, depressed nasal tip, and ears with overfolded helix.
 syndrome (same individual as in Figure 1).">
syndrome (same individual as in Figure 1).">Figure 2.
Boy age seven years with Pitt-Hopkins syndrome (same individual as in Figure 1). Note prominence of the lower face with a well-developed chin. He has a cheerful disposition, deeply set eyes, prominent nasal bridge with depressed nasal tip, and wide mouth (more...)
 syndrome (same individual as in Figures 1 and 2).">
syndrome (same individual as in Figures 1 and 2).">Figure 3.
Boy age 13 years with Pitt-Hopkins syndrome (same individual as in Figures 1 and 2). Note wide nasal ridge and alae, short philtrum, and full lips, with everted lower lip.
 syndrome.">
syndrome.">Figure 4.
Girl age ten years with Pitt-Hopkins syndrome. Note deep-set eyes and depressed nasal tip.
 syndrome (same individual as in Figure 4).">
syndrome (same individual as in Figure 4).">Figure 5.
Woman age 24 years with Pitt-Hopkins syndrome (same individual as in Figure 4). Note prominent nose with depressed tip, short philtrum, full lips, wide mouth with downturned corners, and well-developed chin.
- Deeply set eyes with prominent supraorbital ridges
- Mildly upslanted palpebral fissures
- Broad nasal root, wide nasal ridge, and wide nasal base with enlarged nostrils
- Overhanging or depressed nasal tip, which may be pointed
- Short philtrum
- Thick vermilion of the lower lip, which is often everted
- In some individuals, wide mouth with downturned corners and exaggerated Cupid's bow or tented vermilion of the upper lip
- Widely spaced teeth
- Prominence of the lower face with a well-developed chin. With age, the lower face becomes more prominent and facial features may coarsen.
- Mildly cupped ears with overfolded helices
Episodic hyperventilation and/or breath-holding while awake. Unusual episodes of hyperventilation (which may be followed by apnea) may occur while awake. When present, this finding (in combination with the developmental history and characteristic facial features) is highly suggestive of PTHS; however, the absence of a breathing abnormality should not eliminate consideration of the diagnosis of PTHS as the breathing abnormality may begin sometime in the second half of the first decade, later, or not at all [Zweier et al 2008, Marangi et al 2011].
Note: Information about the presence of the breathing abnormality must be elicited directly: parents may not realize the diagnostic importance of this finding as it would not typically prompt them to seek medical attention [Takano et al 2011; Author, personal observation].
Establishing the Diagnosis
The diagnosis of PTHS is established in a proband with severe intellectual disability and severe speech impairment and confirmed by identification on molecular genetic testing of a heterozygous pathogenic variant in TCF4 or a deletion of the chromosome region in which TCF4 is located (18q21.2) (see Table 1). Rare individuals with a TCF4 pathogenic variant or deletion may lack obvious characteristic facial features, but still manifest intellectual disability and speech impairment.
Molecular genetic testing approaches can include a combination of gene-targeted testing (single-gene testing or a multigene panel) and genomic testing (comprehensive genomic sequencing) depending on the phenotype.
Gene-targeted testing requires that the clinician determine which gene(s) are likely involved, whereas genomic testing does not. Because the phenotype of PTHS is broad, individuals with the distinctive findings described in Suggestive Findings are likely to be diagnosed using gene-targeted testing (see Option 1), whereas those with a phenotype indistinguishable from many other inherited disorders with intellectual disability / developmental delay are more likely to be diagnosed using genomic testing (see Option 2).
Option 1
When the phenotypic and laboratory findings suggest the diagnosis of PTHS, molecular genetic testing approaches can include single-gene testing or use of a multigene panel:
- Single-gene testing. Sequence analysis of TCF4 is performed first and followed by gene-targeted deletion/duplication analysis if no pathogenic variant is detected on sequence analysis.
- A multigene panel that includes TCF4 and other genes of interest (see Differential Diagnosis) may also be considered. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview; thus, clinicians need to determine which multigene panel is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.For this disorder a multigene panel that includes deletion/duplication analysis as well as sequencing is recommended (see Table 1).For an introduction to multigene panels click here. More detailed information for clinicians ordering genetic tests can be found here.
Note: A karyotype should be considered if a diagnosis of PTHS is strongly suspected with normal results on molecular testing, since a chromosome rearrangement disrupting TCF4 without a deletion has been detected in an individual with features of PTHS [Kalscheuer et al 2008, Marangi et al 2011].
Option 2
When the phenotype is indistinguishable from many other inherited disorders with intellectual disability / developmental delay, molecular genetic testing approaches can include comprehensive genomic testing (recommended) and/or gene-targeted testing (multigene panel; to consider):
- Comprehensive genomic testing (when clinically available) includes exome sequencing and genome sequencing.For an introduction to comprehensive genomic testing click here. More detailed information for clinicians ordering genomic testing can be found here.
- A multigene panel for intellectual disability / developmental delay may also be considered.
For each of these options, it would be important to pursue a chromosome microarray (CMA) or a multigene panel that also includes deletion/duplication analysis of TCF4 given the frequency of deletions in this disorder.
Table 1.
Molecular Genetic Testing Used in Pitt-Hopkins Syndrome
| Gene 1 | Method | Proportion of Probands with a Pathogenic Variant 2 Detectable by Method |
|---|---|---|
| TCF4 | Sequence analysis 3 | 70% 4 |
| Gene-targeted deletion/duplication analysis or chromosomal microarray 5 | 30% 6, 7 | |
| Karyotype | See footnote 8 |
- 1.
See Table A. Genes and Databases for chromosome locus and protein.
- 2.
See Molecular Genetics for information on allelic variants detected in this gene.
- 3.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Pathogenic variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 4.
Whalen et al [2012]
- 5.
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications.
- 6.
Pathogenic variants include whole- or partial-gene deletions, varying in size from a single exon to ~12 Mb [Whalen et al 2012]. Of these, at least ten reports describe single-exon or partial-gene deletions, detectable only by deletion/duplication analysis [Brockschmidt et al 2007, Rosenfeld et al 2009, Lehalle et al 2011, Whalen et al 2012].
- 7.
Gene-targeted deletion/duplication testing will detect from single-exon to whole-gene deletions; however, breakpoints of large deletions and/or deletion of adjacent genes may not be detected by these methods. Chromosome microarray analysis (CMA) detects a deletion in about one third of individuals with PTHS.
- 8.
Balanced chromosome rearrangements disrupting TCF4 without a deletion have been detected in some individuals with PTHS [Kalscheuer et al 2008, Marangi et al 2011]; thus, chromosome analysis should be considered in those in whom PTHS is a strong possibility but whose molecular testing gave a normal result.
Clinical Characteristics
Clinical Description
Children with Pitt-Hopkins syndrome (PTHS) typically present in the first year of life with hypotonia and developmental delays. Some infants have been described as being quiet and "unusually good" with excessive sleeping [Giurgea et al 2008].
Developmental delay / intellectual disability. Developmental delays are significant and intellectual disability is moderate to severe. Hypotonia can be significant and motor skills are delayed with a mean age of walking of four to six years (range 27 months - 7 years); some affected individuals may walk only with assistance and others do not acquire independent walking skills [Whalen et al 2012]. Those who walk independently often have a wide-based, unsteady gait. Most require orthotics to stabilize the ankle.
Speech is significantly delayed in all individuals with PTHS and excessive drooling is common. Most are nonverbal. Some have developed a few words, but subsequently regressed later in life and become entirely nonverbal. Rare individuals can string words into sentences. Receptive language is generally stronger than expressive language, and many individuals with PTHS are able to understand and follow simple commands. The use of augmentative communication devices has been useful for many individuals, though other individuals lack the fine motor skills to make use of these devices.
Self-care skills are also delayed and few are reported to develop consistent dressing or toileting skills.
Behavioral. PTHS is most commonly associated with autism spectrum disorder (ASD) symptoms, characterized by impairments in communication, behavior, and social interactions. The relationship between autism and PTHS remains ambiguous; however, in a study conducted by Van Balkom et al, the Autism Diagnostic Interview Revised (ADI-R) assessed ten participants with confirmed PTHS diagnoses who scored at or above social, behavioral, and communication criteria for ASD (2 participants did not score above the behavioral domain cutoff value). Six out of ten participants were observed to have fixations that prompted repetitive exposure to a specific object, listening to the same song, or viewing the same video repeatedly [Van Balkom et al 2012].
Many individuals with PTHS are described as having a happy disposition [Zweier et al 2008, Marangi et al 2011], though it has been proposed that an anxious disposition with a smiling appearance is a more apt description [Whalen et al 2012]. Others are described as being difficult to handle as they develop outbursts of aggression or bouts of shouting associated with frustration or unanticipated changes in routine [Andrieux et al 2008, Giurgea et al 2008, de Pontual et al 2009]. The onset of puberty can be associated with increased aggression and behavioral issues [Authors, personal observation].
Individuals with PTHS may be shy or anxious in new situations and self-aggression can occur. Disruptions of routine have also been associated with episodes of anxiety. Hand biting and head banging have been commonly reported by families of individuals with PTHS.
Unprovoked laughter may occur. Sleep disturbance in childhood is reported in fewer than half of affected individuals [Whalen et al 2012] and includes difficulty falling asleep, problems sleeping through the night, and night terrors [de Winter et al 2016].
Stereotypic head movements (e.g., head rotation) and stereotypic hand movements (e.g., flapping, clapping, washing movements, hand to mouth, finger crossing) are commonly observed [Takano et al 2010, Marangi et al 2011, Whalen et al 2012]. Three individuals have been reported to have lost the use of hand skills [Zweier et al 2008, Armani et al 2012, Whalen et al 2012].
Individuals with PTHS invariably have a profound affinity for music. Families report that during episodes of anxiety or frustration, music will very often soothe or placate them. Many appear to love playing with water, and exhibit a fascination with running water [Authors, personal observation].
Craniofacial. In addition to the craniofacial features discussed in Diagnosis, cherry red lips have been noted infrequently [Ghosh et al 2012].
Growth. Growth is typically in the normal range for size at birth; slower postnatal growth is noted in about 25%.
Head growth was found to slow postnatally in 26% [de Winter et al 2016], with microcephaly reported in up to 60% [Zweier et al 2008, Goodspeed et al 2018].
The initiation and progression of secondary sexual characteristics appear to be unimpaired in individuals with PTHS [Authors, personal observation].
Respiratory. Episodic hyperventilation often followed by breath-holding/apnea while awake are reported in 40%-60% of individuals [Whalen et al 2012, de Winter et al 2016, Goodspeed et al 2018]. Hyperventilation is often associated with anxiety or excitement [Giurgea et al 2008, Steinbusch et al 2013] and is not present during sleep [Maini et al 2012]. Breath-holding may be associated with cyanosis, and in rare cases syncope, and can occur independently of hyperventilation [Whalen et al 2012]. Finger clubbing and chronic hypoxia has been noted in 7%-19% [Whalen et al 2012, Goodspeed et al 2018].
Breathing abnormalities tend to develop between ages three and seven years; few individuals are affected at birth. In one study 30% had symptoms by age 15 years [de Winter et al 2016]. In some children, hyperventilation and/or breath-holding episodes are observed for only a few months or years. However, in most affected individuals breathing abnormalities persist to some degree, though too few adults have been described to make conclusive statements about frequency or severity. Breath-holding/apnea episodes are not typically related to seizure activity [Maini et al 2012]. Treatment can mitigate the severity and frequency of symptoms.
Neurologic. Seizures, reported in 40%-50% of individuals with PTHS, vary in type and severity. Onset of seizures is reported from early infancy to as late as age 18 years [de Pontual et al 2009].
In an internet questionnaire study of 101 families, 37.6% had epilepsy [de Winter et al 2016]. Of those with a history of epilepsy, 23.7% became seizure-free at a mean age of 6.4 years. The majority (51.4%) had involuntary motor movements or tonic-clonic seizures often with absence seizures, 16.2% had only absence seizures, and the rest could not accurately describe their family member's seizures. Since this was a questionnaire study, it is unclear whether "absence" events were true absence seizures or focal-onset seizures with no motor component.
The breathing abnormality in PTHS does not appear to be a manifestation of seizure activity [Maini et al 2012], though in this study, 7/38 individuals with seizures had apnea or hyperventilation shortly before their seizures.
In most individuals with seizures, these are well controlled by antiepileptic drugs – valproic acid, levetiracetam, lamotrigine, and carbamazepine being the most common [de Winter et al 2016].
EEG and MRI findings reported in PTHS are limited.
- One study reported EEGs in four individuals with PTHS; no specific patterns other than occipital and central delta waves were found in the younger two individuals. Pseudoperiodic complexes were present during wakefulness over the central and occipital regions, at times admixed with slow spike and wave in the two older individuals [Amiel et al 2007].
- In those individuals who have had a brain MRI, a variety of findings have been reported, though many studies are reported as normal. The most common findings include white matter abnormalities such as hypoplasia/agenesis of the corpus callosum and white matter hyperintensity in the temporal poles, as well as ventricular dilatation, posterior fossa abnormalities, small hippocampi, and moderate hypoplasia of the frontal lobes [Amiel et al 2007, Marangi et al 2011, Whalen et al 2012].
Signs of general autonomic dysfunction have been observed in some individuals or reported by families [Authors, personal observation]. These include:
- High pain tolerance (very common);
- Lack of tears;
- Impaired ability to sweat and dysregulation of body temperature homeostasis.
Eyes. Myopia, strabismus, and/or astigmatism are present in 50%-60%. Myopia can be severe (>6 diopters) and evident before age two years [Giurgea et al 2008, Stavropoulos et al 2010].
Gastrointestinal. Early feeding issues may occur, although most resolve with age.
Constipation is common (75%) and may be severe. Hirschsprung disease is rare, with one individual reported [Peippo et al 2006].
Gastroesophageal reflux is reported in fewer than half of individuals with PTHS [de Winter et al 2016]. In some individuals, reflux is associated with symptoms of impaired swallowing, and videofluoroscopic swallowing studies may be useful.
Musculoskeletal. Minor hand and foot anomalies such as slender or small hands and feet, broad fingertips, clinodactyly, tapered fingers, transverse palmar crease, flat feet with hindfoot valgus deformity, overriding toes, and short metatarsals have been reported. Absent flexion creases of the thumbs may occur with thumb ankylosis. In one individual an absent thumb tendon was found during surgery [Authors, personal observation].
Hands and feet are reported to be cold and cyanosed in some individuals.
Hyperpronation of the ankles is a nearly universal feature.
Clubfeet have been reported in 20% [Whalen et al 2012].
Scoliosis has been noted in about 25% although no information regarding severity is available.
Skin. Many have had prominent pads on the fingertips and/or toes (persistent fetal pads) [Lehalle et al 2011].
Supernumerary nipples have been observed in at least ten individuals [Rosenfeld et al 2009, Takano et al 2010, Marangi et al 2011].
Adult-onset features. Relatively few older teens or adults have been reported with PTHS; thus it is not yet known what, if any, other adult-onset findings may be of concern in PTHS. Several adults have had significant hand tremors. Another had chronic urinary retention requiring intermittent catheterization [Authors, personal observations].
Other. Hodgkin lymphoma has been reported in an individual age 29 years with PTHS [Zweier et al 2007]. While TCF4 has a role in lymphocyte development [Zhuang et al 1996], the lack of other individuals with PTHS reported to have lymphoma suggests that this finding is coincidental.
Genotype-Phenotype Correlations
According to most studies reported, the phenotype associated with PTHS appears to be independent of TCF4 variant type.
Size of deletion. In several studies comparing chromosomal deletions of varying lengths of chromosome 18q21.2 involving TCF4, individuals appeared to have similar phenotypes to each other and single-nucleotide variants in TCF4 [Andrieux et al 2008, Hasi et al 2011, Stavropoulos et al 2010, Giurgea et al 2008]. However, one case suggested that haploinsufficiency of the neighboring genes MBD1 and MBD2 may confer a more severe Rett syndrome-like phenotype [Kato et al 2010].
Mosaicism for chromosome 18q21.1 deletions have been described. Two individuals with mosaicism for chromosome 18q21.2 deletions (11.9 Mb and 7.5 Mb in size) demonstrated the typical PTHS phenotype [Giurgea et al 2008, Stavropoulos et al 2010]. Another individual with mosaic 18q21.2 deletion (185 kb) involving a portion of TCF4 was reported to have dysmorphic features, attention-deficit disorder, anxiety, self-mutilation, and stiff joints [Pham et al 2014]. Two other individuals mosaic for 18q21 deletions including TCF4 have been described with dysmorphic facial features (though not classic for PTHS), severe developmental delay, and microcephaly. One of these individuals had 5%-10% mosaicism and the other about 40% [Rossi et al 2012].
Penetrance
Penetrance of a pathogenic TCF4 allele is complete, though some phenotypic variability is seen among sibs born to a presumably mosaic parent.
Prevalence
Overall prevalence of PTHS is unknown. However, based on a lower relative detection rate as compared to Williams syndrome and Smith-Magenis syndrome deletions, one laboratory estimated that the frequency of chromosome 18q21 deletions associated with PTHS is between 1:34,000 and 1:41,000 [Rosenfeld et al 2009]. If deletions are found in approximately one third of individuals with PTHS, the frequency of the condition could be as high as 1:11,000.
PTHS occurs in both males and females and is not limited to any specific ethnic background.
Differential Diagnosis
See Table 2 for disorders with features that overlap those of Pitt-Hopkins syndrome (PTHS).
Table 2.
Disorders to Consider in the Differential Diagnosis of Pitt-Hopkins Syndrome
| Disorder | Gene(s) | MOI | Clinical Features of the Differential Diagnosis Disorder | |
|---|---|---|---|---|
| Overlapping w/PTHS | Distinguishing from PTHS | |||
| Mowat-Wilson syndrome | ZEB2 | AD | DD, absent speech, hypotonia w/some overlap in facial features | Unusual uplifted earlobe configuration & hypertelorism; more likely to be associated w/variety of malformations (Hirschsprung disease, genitourinary anomalies, heart defects, structural eye anomalies); absence of PTHS facial features |
| Angelman syndrome (AS) | UBE3A | AD | DD w/absent speech, seizures, microcephaly, wide-based gait, happy disposition; 2% of 86 individuals suspected of having AS actually had PTHS w/a TCF4 pathogenic variant. | EEG almost universally abnormal w/slow background & rhythmic delta waves & "posterior spike" patterns; seizures in 80%-95% of individuals; sleep problems common; tremor & ataxia almost universal; absence of PTHS facial features |
| Joubert syndrome (JS) | Various; >20 reported 1 | AR (XL) 2 | Hypotonia, DDs, episodic tachypnea &/or apnea | Distinctive cerebellar vermis hypoplasia associated w/molar tooth sign on brain MRI; oculomotor apraxia; & truncal ataxia; breathing abnormalities of JS noted in early infancy & improving w/age; absence of PTHS facial features |
| Rett syndrome | MECP2 | XL | Episodic hyperventilation/ apnea | Normal early development followed by stagnation, then regression of language & motor skills; progressive; seen only in females; absence of PTHS facial features |
| ARX-related ID syndrome (OMIM 309510) | ARX | XL | DD, severe speech disorder, seizures; recurrent hyperventilation episodes reported in 1 individual w/an ARX pathogenic variant | Hand & lower-limb dystonia; absence of characteristic PTHS facial features |
| NRXN1-associated AR ID disorder (OMIM 614325) | NRXN1 | AR | Severe global delays, lack of speech, stereotypies, episodic breathing abnormalities in 3 individuals (2 w/hyperventilation episodes;1 w/breath-holding episodes) | Absence of characteristic PTHS facial features; abnormal sleep-wake cycles (not commonly reported w/PTHS) |
| CNTNAP2-associated AR ID disorder (OMIM 610042) | CNTNAP2 | AR | Severe global delays, lack of speech, stereotypies, seizures, episodic hyperventilation episodes reported in 3 individuals | Absence of characteristic PTHS facial features |
| WAC-related ID | WAC | AD | DD, certain common facial features (e.g., deep-set eyes, prominent lower face), anxiety, ASD, constipation, visual problems, seizures; sleep disturbances | Neonatal feeding problems; ID less severe; breathing abnormalities not typically hyperventilation/apnea |
AD = autosomal dominant; AR = autosomal recessive; ASD = autism spectrum disorder; DD = developmental delay; ID = intellectual disability; MOI = mode of inheritance; XL = X-linked
- 1.
See Joubert Syndrome to view genes associated with this phenotype.
- 2.
Joubert syndrome is inherited predominantly in an autosomal recessive manner. Joubert syndrome caused by pathogenic variants in OFD1 is inherited in an X-linked manner. Digenic inheritance has been reported.
Management
Evaluations Following Initial Diagnosis
To establish the extent of disease and needs of an individual diagnosed with Pitt-Hopkins syndrome (PTHS), the following evaluations are recommended if they have not already been completed:
- Developmental assessment to establish a cognitive baseline and determine the types of services and educational strategies needed. In school-aged children this information is important for the child's individualized education plan (IEP).
- Assessment for the use of nonverbal communication devices and strategies (given that most individuals do not develop useful speech)
- Child behavior specialist to aid with behavioral concerns. Consultation with a child psychiatrist may be warranted if there are significant behavioral issues.
- Pulmonary consultation if history of respiratory pattern abnormality is identified on directed questioning. Polysomnography may be indicated if there is a history of episodic apnea, especially when associated with cyanosis (typically alternating with episodes of hyperventilation).
- Child neurology evaluation to establish a neurologic baseline and evaluate for other neurologic issues such as sleep dysfunction or seizures. This should be expedited if there are familial concerns about seizure activity. Consider a baseline polysomnogram to assess for sleep disturbances.
- Ophthalmology evaluation to evaluate for myopia, astigmatism, and/or strabismus
- Gastroenterology evaluation to establish treatment regimen for chronic constipation and other possible GI issues such as gastroesophageal reflux
- Musculoskeletal evaluation by an orthopedist or physiatrist to evaluate ambulatory skills and need for special mobility equipment and/or orthotics to aid in foot position
- Consultation with a clinical geneticist and/or genetic counselor
Treatment of Manifestations
Developmental Delay / Intellectual Disability Management Issues
The following information represents typical management recommendations for individuals with developmental delay / intellectual disability in the United States; standard recommendations may vary from country to country.
Ages 0-3 years. Referral to an early intervention program is recommended for access to occupational, physical, speech, and feeding therapy. In the US, early intervention is a federally funded program available in all states.
Ages 3-5 years. In the US, developmental preschool through the local public school district is recommended. Before placement, an evaluation is made to determine needed services and therapies and an IEP is developed.
Ages 5-21 years
- In the US, an IEP based on the individual's level of function should be developed by the local public school district. Affected children are permitted to remain in the public school district until age 21.
- Discussion about transition plans including financial, vocation/employment, and medical arrangements should begin at age 12 years. Developmental pediatricians can provide assistance with transition to adulthood.
All ages. Consultation with a developmental pediatrician is recommended to ensure the involvement of appropriate community, state, and educational agencies and to support parents in maximizing quality of life.
Consideration of private supportive therapies based on the affected individual's needs is recommended. Specific recommendations regarding type of therapy can be made by a developmental pediatrician.
In the US:
- Developmental Disabilities Administration (DDA) enrollment is recommended. DDA is a public agency that provides services and support to qualified individuals. Eligibility differs by state but is typically determined by diagnosis and/or associated cognitive/adaptive disabilities.
- Families with limited income and resources may also qualify for supplemental security income (SSI) for their child with a disability.
Motor Dysfunction
Gross motor dysfunction
- Physical therapy is recommended to maximize mobility and to reduce the risk for later-onset orthopedic complications (e.g., contractures, scoliosis, hip dislocation).
- Consider use of durable medical equipment as needed (e.g., wheelchairs, walkers, bath chairs, orthotics, adaptive strollers).
- For muscle tone abnormalities including hypertonia or dystonia, consider involving appropriate specialists to aid in management of baclofen, Botox®, anti-parkinsonian medications, or orthopedic procedures.
Fine motor dysfunction. Occupational therapy is recommended for difficulty with fine motor skills that affect adaptive function such as feeding, grooming, dressing, and writing.
Oral motor dysfunction. Assuming that the individual is safe to eat by mouth, feeding therapy – typically from an occupational or speech therapist – is recommended for affected individuals who have difficulty feeding due to poor oral motor control.
Communication issues. Strong consideration should be given to early training with alternative means of communication (e.g., Augmentative and Alternative Communication [AAC]) for those with PTHS, given the presence of severe expressive language difficulties.
Social/Behavioral Concerns
Children may qualify for and benefit from interventions used in treatment of autism spectrum disorder, including applied behavior analysis (ABA). ABA therapy is targeted to the individual child's behavioral, social, and adaptive strengths and weaknesses and is typically performed one on one with a board-certified behavior analyst.
Consultation with a developmental pediatrician may be helpful in guiding parents through appropriate behavior management strategies or providing prescription medications when necessary.
Concerns about serious aggressive or destructive behavior can be addressed by a pediatric psychiatrist.