Autosomal Erythropoietic Protoporphyria
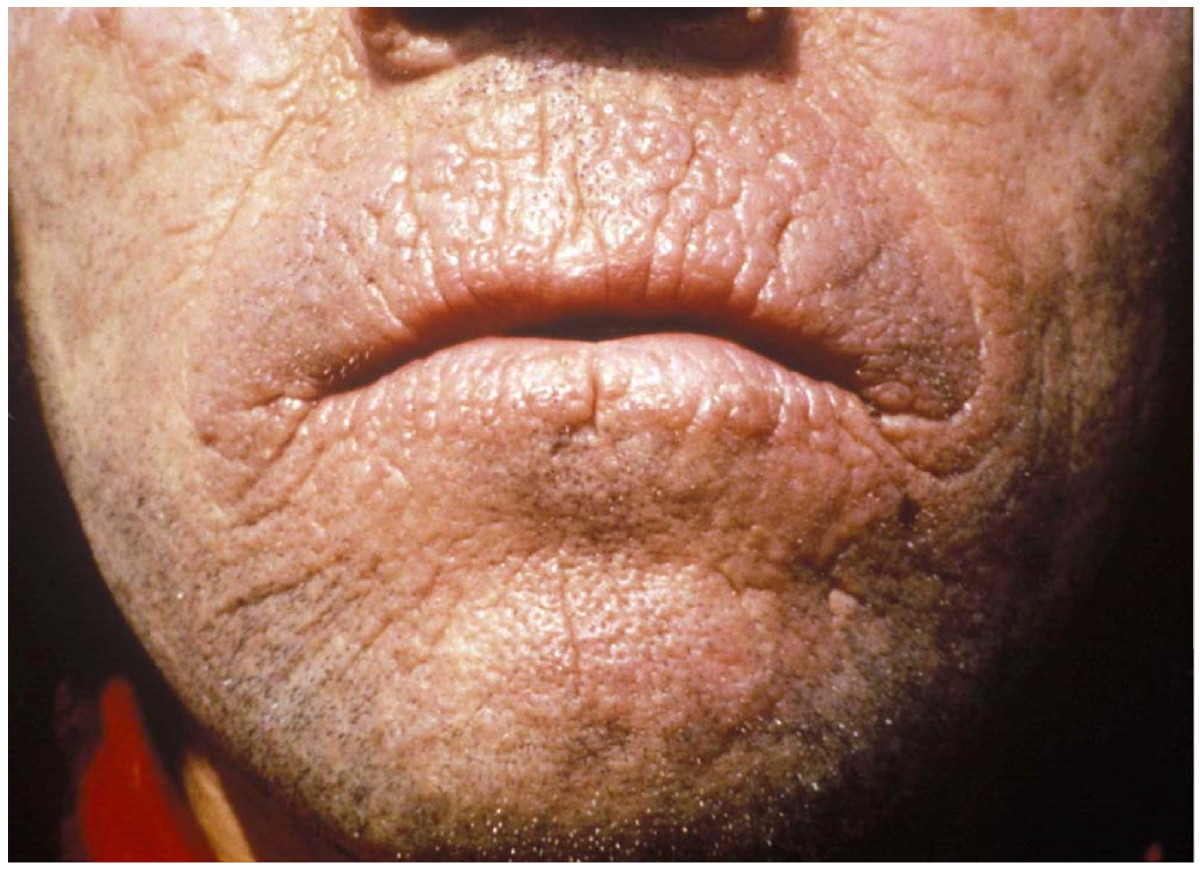
Erythropoietic protoporphyria (EPP) is a type of porphyria. Porphyrias are caused by an abnormality in the heme production process. Heme is essential in enabling our blood cells to carry oxygen and in breaking down chemical compounds in the liver. Erythropoietic protoporphyria is caused by pathogenic variants (mutations) in the FECH gene which lead to an impaired activity of ferrocheletase (FECH), an important enzyme in heme production. This results in the build-up of protoporphyrin in the bone marrow, red blood cells, blood plasma, skin, and eventually liver. Build up of protoporphyrin can cause extreme sensitivity to sunlight, liver damage, abdominal pain, gallstones, and enlargement of the spleen. Inheritance is autosomal recessive.
Treatment includes avoiding sun and UV light exposure, vitamin D supplements, creams for tanning, and using protective clothing. A medication known as Afamelanotide (Scenesse®), a synthetic α-melanocyte stimulating hormone (a melanocyte is a skin cell that produces melanin, a skin-darkening pigment) analog was approved for treatment of EPP by the European Medicines Agency in 2014 and is awaiting approval in United States by the FDA. This medication increases pain-free sun exposure and has improved quality of life in those with EPP. Liver complications may be treated with cholestyramine and other porphyrin absorbents , plasmapheresis, a procedure where the liquid part of the blood, or plasma, is separated from the blood cells, and intravenous heme are sometimes beneficial. Liver transplantation may be required.
Another type of porphyria, known as X-linked protoporphyria, is caused by a variation in the ALAS2 gene and have similar symptoms to erythropoietic protoporphyria when males are affected by EPP.
Treatment includes avoiding sun and UV light exposure, vitamin D supplements, creams for tanning, and using protective clothing. A medication known as Afamelanotide (Scenesse®), a synthetic α-melanocyte stimulating hormone (a melanocyte is a skin cell that produces melanin, a skin-darkening pigment) analog was approved for treatment of EPP by the European Medicines Agency in 2014 and is awaiting approval in United States by the FDA. This medication increases pain-free sun exposure and has improved quality of life in those with EPP. Liver complications may be treated with cholestyramine and other porphyrin absorbents , plasmapheresis, a procedure where the liquid part of the blood, or plasma, is separated from the blood cells, and intravenous heme are sometimes beneficial. Liver transplantation may be required.
Another type of porphyria, known as X-linked protoporphyria, is caused by a variation in the ALAS2 gene and have similar symptoms to erythropoietic protoporphyria when males are affected by EPP.