Cerebral Arteriopathy, Autosomal Dominant, With Subcortical Infarcts And Leukoencephalopathy, Type 1
A number sign (#) is used with this entry because of evidence that autosomal dominant cerebral arteriopathy with subcortical infarcts and leukoencephalopathy type 1 (CADASIL1) is caused by heterozygous mutation in the NOTCH3 gene (600276) on chromosome 19p13.
DescriptionAutosomal dominant cerebral arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a progressive disorder of the small arterial vessels of the brain manifest by migraine, strokes, and white matter lesions, with resultant cognitive impairment in some patients (review by Kalimo et al., 1999).
Clinical FeaturesStevens et al. (1977) reported an English family with onset of recurrent cerebral ischemic strokes between 39 and 57 years resulting in progressive neurologic dysfunction and eventual dementia. Affected individuals did not have hypertension, diabetes, or increased cholesterol, but neuropathologic investigation showed abnormalities of the cerebral vasculature; the authors suggested that it was a form of 'vascular encephalopathy.' Low et al. (2007) provided a follow-up of the family reported by Stevens et al. (1977), including confirmation of the CADASIL diagnosis by identification of a pathogenic mutation in the NOTCH3 gene (see MOLECULAR GENETICS).
In a family originating from northern France, Mas et al. (1992) described a genetic disorder characterized by recurrent attacks of focal brain dysfunction starting in mid-adulthood and leading in some to severe motor disability with pseudobulbar palsy and dementia of the subcortical type. Neuroimaging evidence of leukoencephalopathy and well-circumscribed lesions consistent with small deep infarcts were found in clinically affected individuals as well as in some asymptomatic members of the family. Although there was an instance of first-cousin marriage in the family, Mas et al. (1992) thought that the pedigree pattern suggested autosomal dominant inheritance. Members of 4 successive generations were thought to be affected. The only instance of male-to-male transmission was from a deceased father who was probably affected and an asymptomatic son who was affected by evidence on neuroimaging. Although Mas et al. (1992) thought their family represented a distinct disorder, it is quite possible that all of these reports related to the same condition. See also the large family reported by Tournier-Lasserve et al. (1991) in which of 45 subjects studied, 9 were clinically affected and 8 others, although clinically asymptomatic, had MRI signs of leukoencephalopathy.
Baudrimont et al. (1993) reported the pathologic findings in 1 of the affected members of the family reported by Tournier-Lasserve et al. (1991). A previously healthy woman was 40 years old when she first experienced a grand mal seizure. Twelve years later she suffered 2 other grand mal seizures and thereafter had recurrent strokes as well as psychiatric disturbances (depression, manic episodes, and dementia). After a stroke at the age of 57, she became tetraplegic with severe pseudobulbar palsy and died 2 years later. Pathologic examination demonstrated a recent capsulolenticular hematoma, multiple small deep infarcts, diffuse myelin loss and pallor of the hemispheric white matter, and a widespread vasculopathy of the small arteries penetrating the white matter. The arterial wall was markedly thickened with an extensive nonamyloid eosinophilic deposit in the media and reduplication of the internal elastic lamella. Baudrimont et al. (1993) concluded that the underlying lesion in this disorder is located in the small arteries. It differs from both arterial sclerosis and amyloid angiopathies but is similar to that described in some cases of hereditary multi-infarct dementia.
Chabriat et al. (1995) used MRI and genetic linkage analysis to study 148 subjects belonging to 7 families. They concluded that 45 family members (23 males and 22 females) were clinically affected. Recurrent subcortical ischemic events (in 84%), progressive or stepwise subcortical dementia with pseudobulbar palsy (in 31%), migraine with aura (in 22%), and mood disorders with severe depressive episodes (in 20%) were the main features. All symptomatic subjects had prominent signal abnormalities on MRI with hyperintense lesions on T2-weighted images in the subcortical white matter and basal ganglia; the same findings were present in 19 asymptomatic subjects. The mean age at onset of symptoms was 45 years (SD = 10.6), with attacks of migraine with aura occurring earlier in life, at a mean age of 38.1 (SD = 8.03) then ischemic events at a mean age 43.9 years (SD = 10.7). The mean age of death was 64.5 (SD = 10.6) years. On the basis of MRI data, the penetrance of the disease appeared complete between 30 and 40 years of age. Genetic analysis showed strong linkage to chromosome 19 in all 7 families, suggesting genetic homogeneity.
Hutchinson et al. (1995) used MRI to study 15 members of an Irish family, 10 of whom had evidence of CADASIL. Five members of this family had hemiplegic migraine. They proposed that hemiplegic migraine (141500) may be an allelic disorder to CADASIL. However, within this pedigree was a 36-year-old woman who did not have CADASIL by MRI criteria and did not have the CADASIL affected alleles in her haplotype. The authors suggested that this anomalous individual was either a double recombinant or that her hemiplegic migraine was a phenocopy, due to an unrelated mechanism.
Verin et al. (1995) studied the clinical features of 20 symptomatic individuals in a 4-generation pedigree with CADASIL. Verin et al. (1995) believed that this pedigree was distinguished from those previously published by the high frequency of migraine in psychotic mood disorders with early neurologic manifestations. They proposed that the natural history of the phenotype could be viewed in 3 stages. Stage 1 (between 20 and 40 years) is characterized by frequent migraine-like episodes, as well as well-delineated lesions of the white matter; stage 2 (between 40 and 60 years) presents with stroke-like episodes, affective disorders, and coalescent lesions of the white matter lacunae of the basal ganglia; and stage 3 is characterized by subcortical dementia and pseudobulbar palsy.
Glusker et al. (1998) presented the clinical, imaging, and neuropathologic data for a family with an autosomal dominant, nonhypertensive, progressive cerebral arteriopathy and leukoencephalopathy. Clinical presentation was characterized by progressive dementia, gait abnormalities, and, in some, Parkinson-like symptoms. MRI abnormalities, consisting of white matter T2 hyperintensities and cystic-appearing T1 hypointensities, were present in 7 family members. The basal ganglia also showed cystic abnormalities. Neuropathologic examination in 2 cases revealed numerous lacunar infarct-like lesions, extensive demyelination, and widespread hyalinization of arteriolar walls with karyolysis and granular deposits within the media. Affected members occurred in 4 generations. The index patient was referred at the age of 52 years for evaluation of suspected multiple sclerosis. He had right-sided hemiparesis, left-central facial weakness, diffuse bradykinesia, retropulsion, truncal ataxia, and dementia. The mother of the index case was referred at age 71 years. She had mild bradykinesia and diffuse hyperreflexia in all 4 extremities. The family was of Central American ancestry.
Dichgans et al. (1998) described the phenotypic spectrum of 102 biopsy-proven cases of CADASIL in 29 German and Austrian families. The most consistent finding was ischemic episodes, usually classic transient ischemic attacks or lacunar strokes, but occasionally insidious deficits that developed over several days. Cognitive deficits were seen in 59%, migraine in 38%, and epilepsy in 10%. Mean age at death was 53.2 +/- 10.9 years for males and 59.3 +/- 8.8 years for females.
Rufa et al. (2004) described a patient with CADASIL whose first symptom of the disorder was acute unilateral visual loss at age 27 years. The patient was diagnosed as having nonarteritic anterior ischemic optic neuropathy (NAION; 258660) caused by infarction of the optic nerve resulting from local vascular impairment. Electrophysiologic studies showed abnormal visual evoked potentials (VEPs) and electroretinogram (ERG) in the affected eye. His affected son had less severe ocular involvement. Rufa et al. (2004) noted that acute ocular involvement in CADASIL is uncommon.
In 5 patients with CADASIL confirmed by genetic analysis, Rufa et al. (2004) found a reduction in optic nerve head, temporal inferior, and temporal superior blood flow and volume compared to controls. None of the 5 patients had visual symptoms at the time of examination, but all showed abnormal electroretinograms, and 3 showed narrowed retinal arteriolar vessels.
Golomb et al. (2004) reported a 14-year-old girl with a 3-year history of severe headaches, 3 episodes of right hemiparesis (at ages 11, 12, and 14 years) with residual symptoms, persistent hypertension, depression, and a mutation in the NOTCH3 gene. She had no relevant family history. Although several cranial MRI studies were normal, Golomb et al. (2004) suggested that the patient had unusually early-onset CADASIL.
Roine et al. (2005) found that 12 (48%) of 25 Finnish mothers with CADASIL caused by the R133C NOTCH3 mutation (600276.0008) experienced neurologic symptoms in 17 of their 43 pregnancies. The most common symptoms were hemiparesthesia (65%), hemiparesis (36%), aphasia (65%), and visual disturbance (47%). In 82% of the patients with complicated pregnancies, the symptoms were the first manifestations of CADASIL, and they were most common during puerperium and in patients older than 30 years. Roine et al. (2005) concluded that CADASIL may be a risk factor for complicated pregnancies and that CADASIL should be considered in the differential diagnosis of neurologically complicated pregnancies.
Peters et al. (2005) conducted a cross-sectional study of 65 NOTCH3 mutation carriers and 30 matched comparison subjects utilizing a series of assessments that included ratings of global cognition, the cognitive portion of the Vascular Dementia Assessment Scale, and specific tests of executive function and attention with measures of processing speed and error monitoring. CADASIL subjects had pronounced impairments of the timed measures as well as measures of error monitoring. Prominent deficits in verbal fluency and ideational praxis were observed, while recall, orientation, and receptive language skills were largely preserved. Peters et al. (2005) noted that this profile of cognitive impairment was present at an early stage.
Saiki et al. (2006) reported a Japanese family in which 6 members had CADASIL caused by a heterozygous mutation in the NOTCH3 gene (600276.0009). All affected individuals had a history of ischemic episodes and variable features of dementia, hemiparesis, urinary incontinence, dysarthria/dysphagia, migraine, and mood disorders. MRI studies showed lacunar infarctions and subcortical white matter changes. No affected individuals had involvement of the anterior temporal lobes. All affected individuals also had varicose veins that developed between ages 14 and 30. Biopsies of varicose veins from 3 individuals showed marked intimal hypertrophy, localized thinning of smooth muscle layers, and infiltrated fibrous tissue. Venous smooth muscle cells were irregularly shaped and contained granular osmiophilic material. Saiki et al. (2006) noted that varicose veins had not previously been reported in CADASIL.
In a study of 27 Korean patients with CADASIL from 9 unrelated families, Kim et al. (2006) found that clinical features were similar to those reported in other populations, except for the relatively uncommon finding of migraine, which occurred in only 8.3% of patients.
Choi et al. (2006) found that 5 (25%) of 20 patients with genetically confirmed CADASIL had intracranial hemorrhages (ICH), and that ICH was the initial neurologic presentation in 2 patients. All patients had hypertension as a risk factor, and all had been taking antiplatelet agents, except for the 2 who presented with ICH; however these factors were not significantly different from CADASIL patients without ICH. Brain MRI results showed a significant correlation between the development of ICH and the number of cerebral microbleeds.
Pantoni et al. (2010) performed a retrospective analysis and comparison of clinical features in a cohort of 81 patients suspected of having CADASIL, including 16 (20%) who had NOTCH3 mutations and 65 without NOTCH3 mutations. Patients with genetically confirmed CADASIL had a higher frequency of migraine (73% vs 39%), stroke before the age of 60 among relatives (71% vs 32%), severe leukoencephalopathy (94% vs 62%), white matter changes in the anterior temporal lobes (93% vs 45%), external capsule involvement (100% vs 50%), and presence of lacunar infarcts (100% vs 65%), compared to those without mutations. However, the frequency of vascular risk factors was similar between the 2 groups, and no feature was peculiar to either group.
Chitnis and Hollmann (2012) reported a 26-year-old man of Portuguese descent who was found to carry a heterozygous NOTCH3 mutation inherited from his affected mother. Before identification of the mutation, his clinical presentation was similar to that of Balo concentric sclerosis, a rare disorder associated with demyelinating diseases, such as multiple sclerosis (126200). He had subacute onset of right-sided hemiparesis and dysmetria, right-sided extensor plantar responses, and aphasia. Brain MRI showed multiple bilateral concentric ring-like structures in the centrum semiovale and the corona radiata on T2 imaging. CSF analysis showed leukocytes and erythrocytes with absent oligoclonal bands. Plasmapheresis exchanges resulted in significant improvement. One year later, his neurologic examination was essentially normal. Chitnis and Hollmann (2012) raised questions about the role of the NOTCH3 mutation in this patient's presentation, which may shed light on disease pathogenesis of both CADASIL and Balo concentric sclerosis. Hypotheses included the idea that Balo may result from mutations predisposing to hypoxic tissue injury, that CADASIL can present as Balo, and that vascular risk factors such as a NOTCH3 mutation may influence the presentation of a primary demyelinating disorder. It was of note that the patient responded well to plasmapheresis, which suggested a demyelinating disorder.
Radiologic Findings
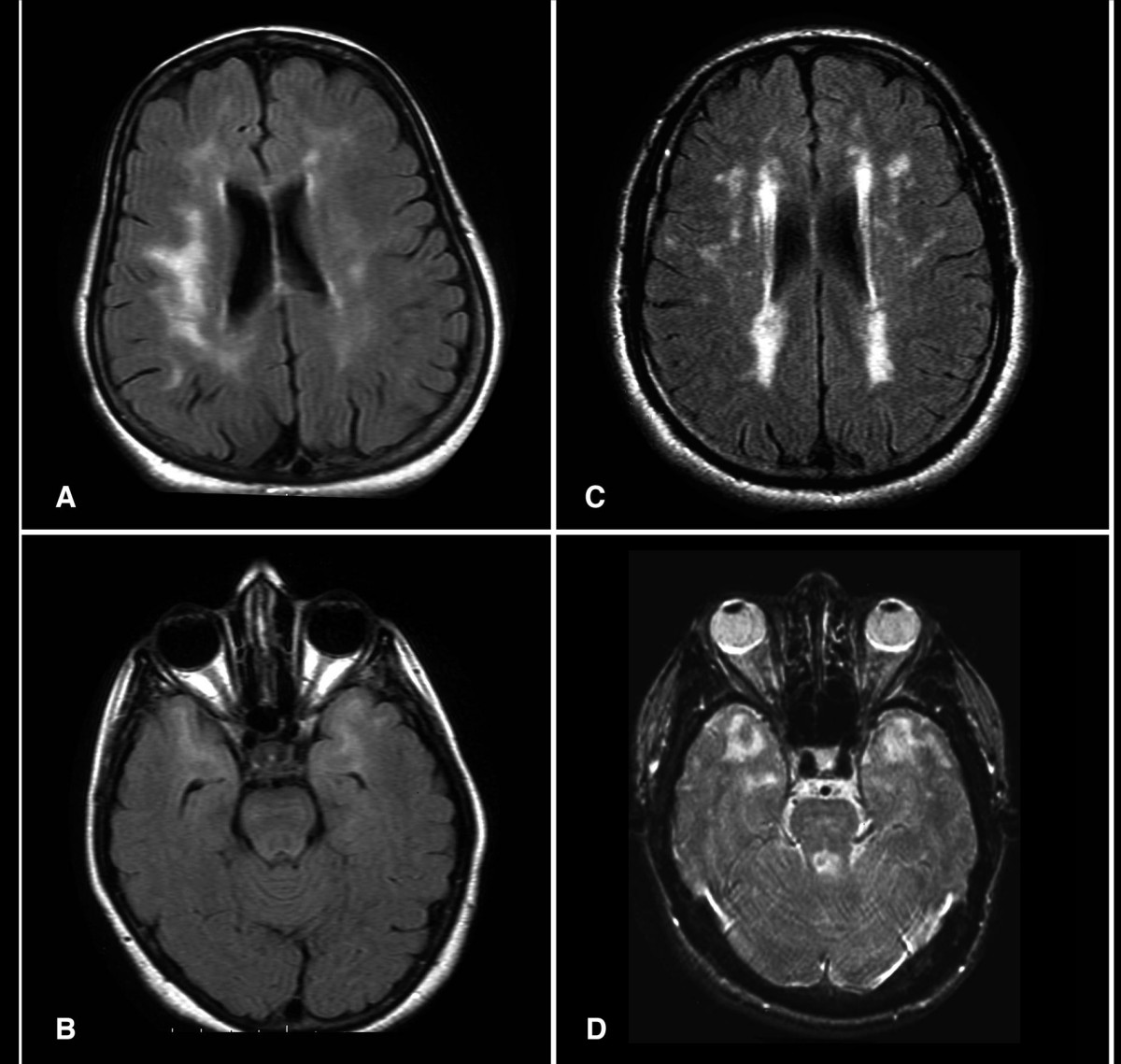
Skehan et al. (1995) studied the MRI appearance in 10 individuals in one large Irish autosomal dominant family and found 2 major types of abnormalities. The most striking were large confluent patches of high-signal change on T2- and proton density-weighted images present throughout the white matter, especially in the anterior part of the temporal lobes and the periventricular portion of the occipital lobes. Additionally, they detected small linear and punctate lacunes present not only in the periventricular white matter but also in the brain stem, basal ganglia, thalamus, external capsule, and corpus callosum.
Among 6 individuals under the age of 35 years who carried a mutation in the NOTCH3 gene, Lesnik Oberstein et al. (2003) found an increase in white matter hyperintensities on brain MRI compared to controls. The lesions showed a characteristic pattern in the anterior temporal lobes, the frontal lobes, and the periventricular caps. Although there was no physical or cognitive impairment in the 6 mutation carriers, migraine with aura was more common than in the controls.
Van den Boom et al. (2003) reported MRI findings in 40 CADASIL patients ranging in age from 21 to 59 years. All 5 patients younger than 30 years of age were clinically asymptomatic. All patients, including those who were asymptomatic, demonstrated hyperintense lesions on T2-weighted MRI. In patients aged 20 to 30 years, supratentorial hyperintense lesions were generally found in the frontal and anterior temporal lobe. About 20% of younger patients also developed subcortical lacunar lesions. After age 30 years, patients developed hyperintense lesions and lacunar infarcts in infratentorial structures, the basal ganglia, and the thalamus, as well as in periventricular regions. In addition to progression of the other lesions, patients over 40 years developed microbleeds, which were usually smaller than 5 mm. Lacunar infarcts were demonstrated in the brainstem in older patients. In general, there was a clear progression of lesions with age.
Using serial brain imaging in 76 patients with CADASIL over 2 years, Peters et al. (2006) identified brain atrophy as an important aspect of the disease process and established significant correlations with disability and global cognitive performance. The mean annualized rate of brain volume loss was 0.56%, which is 2 times higher than that of healthy age-matched subjects. Age, male sex, and increased systolic blood pressure were the main risk factors for lower brain volume.
By brain MRI of 147 patients with CADASIL, Viswanathan et al. (2007) observed a correlation between T1-weighted hypointense lacunar lesions and global cognitive dysfunction. In contrast, T2-weighted hyperintense lesions, representing subcortical white matter lesions, and cerebral microhemorrhages had no independent influence on cognitive function. Disability was associated with age, volume of lacunar lesions, cerebral microhemorrhages, and systolic blood pressure, but not with white matter hyperintensities. The findings implicated lacunar lesions as having an important clinical impact on patients with CADASIL.
MappingThrough analysis of genetic linkage in 2 unrelated French families, including a family reported by Mas et al. (1992), Tournier-Lasserve et al. (1993) mapped the disease locus (CASIL) to 19q12. St Clair et al. (1995) used polymorphic DNA markers spanning the 19q12 region in linkage studies in a large Scottish pedigree with neuropathologically confirmed hereditary multi-infarct dementia; linkage was excluded. Ragno et al. (1995) reported an Italian kindred in which at least 16 subjects had either symptomatic or asymptomatic MRI evidence of a small vessel leukoencephalopathy. Linkage analysis with a marker for D19S226 at theta = 0.05 gave a maximum lod score of 3.660. Neuropathologic examination of 1 subject from this kindred demonstrated widespread vasculopathy of the perforating arterials, characterized by deposition of eosinophilic-congophilic material that did not immunostain with antibodies against prion protein, beta amyloid (104760), cystatin C (604312), transthyretin (176300), or HSP70 (140550).
To the original 2 large French families in which linkage of CADASIL to a 12-cM interval on chromosome 19 was demonstrated, Ducros et al. (1996) added 13 more families, including a total of 199 potentially informative meioses, and did genotyping with 8 polymorphic markers located between flanking markers D19S221 and D19S215 identified in the earlier study. All families were linked to chromosome 19. The highest combined lod score (maximum lod = 37.24 at theta = 0.01) was obtained with marker D19S841. This narrowed the mapping to a 2-cM interval on 19p13.1. The data strongly supported genetic homogeneity of this condition and established the value of its clinical and neuroimaging diagnostic criteria. Strong evidence of linkage to 19p was also found by Sabbadini et al. (1995) in an Italian CADASIL pedigree. Ducros et al. (1996) suggested that the negative results of St Clair et al. (1995) cannot be taken as proof of genetic heterogeneity since the scoring of pedigree members for linkage analysis was not established on the basis of MRI for many family members, leading to a high risk of misclassification and therefore false recombinants. Ducros et al. (1996) pointed out that paroxysmal cerebellar ataxia (108500) and familial hemiplegic migraine (141500) map to the same region, 19p13.
Dichgans et al. (1996) identified a crossover in a clinically affected family member which helped to refine the localization of the CADASIL locus to an 8-cM interval bracketed by D19S226 and D19S222. From the previous location of the gene involved in familial hemiplegic migraine, it was concluded that these disorders are probably not allelic.
Molecular GeneticsJoutel et al. (1996) characterized the human NOTCH3 gene, which they mapped to the CADASIL critical region. Furthermore, they identified mutations of the NOTCH3 gene (e.g., 600276.0001) in CADASIL patients that caused serious disruption of NOTCH3, suggesting that mutations in this gene are the cause of the disorder.
Filley et al. (1999) reported a 62-year-old man who had a slowly progressive 25-year history of personality change, psychosis, mood disorder, and dementia. He showed minimal motor impairment, as well as a deficit in sustained attention, slowed information processing, impaired learning with intact recognition, a mild visuospatial deficit, and frontal lobe dysfunction. MRI of the brain revealed extensive leukoencephalopathy. Right frontal brain biopsy showed ill-defined white matter pallor. Granular osmiophilic material adjacent to vascular smooth muscle cells on electron microscopy of a skin biopsy and an arg169-to-cys mutation (600276.0002) in the EGF domain of the NOTCH3 gene (600276) established the diagnosis of CADASIL. The authors concluded that the dementia that occurs in CADASIL closely resembles that which may occur in other white matter disorders.
Arboleda-Velasquez et al. (2002) reported a Colombian kindred with CADASIL characterized by early-onset stroke (median age, 31 years), migraine with aura, and confluent MRI hyperintensities. They identified a heterozygous 1441T-C transition in exon 8 of the NOTCH3 gene, resulting in a cys455-to-arg (C455R; 600276.0006) substitution. The mutation abolishes the fourth cysteine residue at EGF-like repeat 11 and may affect the interaction of the NOTCH3 receptor with its ligands. Despite the early onset of stroke, all patients had relatively well-preserved cognitive and functional status more than 2 decades after onset.
In affected members of an English family with CADASIL (Stevens et al., 1977), Low et al. (2007) identified a heterozygous missense mutation in the NOTCH3 gene (R141C; 600276.0018). The cerebral microvasculature of affected individuals showed intense accumulation of NOTCH3 N-terminal fragments.
Genotype/Phenotype CorrelationsScheid et al. (2008) reported a German family in which 3 individuals had a relatively mild variant of CADASIL. The index patient had episodic headache, vertigo, paresthesias, weakness, and cognitive decline in her forties. She also had sensorineural hearing loss and arterial hypertension. Brain MRI showed widespread white matter lesions. Two affected relatives at ages 71 and 63, respectively, had very mild neurologic signs, including nystagmus and hearing loss, associated with variable severity of white matter lesions. An additional unrelated patient had clinical disease apparent in her seventies. All patients had the same cysteine-sparing mutation in the NOTCH3 gene (A1020P; 600276.0010), which the authors postulated may have resulted in the milder phenotype of later symptom onset and later onset of MRI changes. Scheid et al. (2008) also suggested that sensorineural hearing loss may be an additional manifestation of the disorder.
From a clinical and genetic study in 2 unrelated families, Rutten et al. (2013) provided evidence that loss-of-function NOTCH3 mutations do not cause CADASIL. In the first family, a 55-year-old man with polyneuropathy, migraine with aura, and ischemic strokes between ages 50 and 52 was found to carry a heterozygous truncating variant in the NOTCH3 gene (R103X). Brain MRI showed old large vessel infarctions, but no white matter changes consistent with CADASIL. Skin biopsy was negative for NOTCH3 staining, but there was normal structure of the vessel wall and no electron microscopic deposits characteristic of the disorder. The patient's 50-year-old brother also carried the NOTCH3 variant, but was asymptomatic with a normal brain MRI; family history was negative for stroke and dementia. In a second family, a patient with classic MRI findings of CADASIL was compound heterozygous for a tyr710-to-cys (Y710C) mutation in the NOTCH3 gene and an intragenic frameshift deletion. The Y710C mutation was inherited from his possibly affected mother who had transient functional deficit of the arm at age 40 years without available brain imaging, and the deletion was inherited from his unaffected father whose skin biopsy was negative for CADASIL. Most CADASIL-associated NOTCH3 mutations alter conserved cysteine residues and are postulated to cause a toxic neomorphic effect. Rutten et al. (2013) concluded that hypomorphic NOTCH3 mutations do not cause CADASIL, which has important implications for diagnostic interpretation.
Population GeneticsMykkanen et al. (2004) performed haplotype analysis in 60 patients from 18 Finnish CADASIL families with an arg133-to-cys mutation in the NOTCH3 gene (R133C; 600276.0008). Using 10 microsatellite markers, the authors found a similar haplotype linked to the mutation in all 18 pedigrees, indicating a single common ancestor for all of the Finnish R133C families. Age analysis of the founder mutation placed the introduction of the mutation in the late 1600s or early 1700s.
DiagnosisSchroder et al. (1995) demonstrated pathologic findings on sural nerve biopsy in individuals from a family with 4 affected members in 3 successive generations. Light microscopic abnormalities were very mild. However, by electron microscopy, they were able to demonstrate characteristic electron dense, extracellular granular deposits in close association with smooth muscle cells and to a lesser degree with pericytes and endothelial cells. They found focal pinocytotic vesicles at the site of close contact between the extracellular deposit and the surface membrane of the smooth muscle cell, suggesting exocytosis of abnormal material. The authors stated that this was the first demonstration that the diagnosis of CADASIL could be verified by sural nerve biopsy.
Joutel et al. (2001) established whether immunostaining skin biopsy samples with a monoclonal antibody specific for NOTCH3 could form the basis of a reliable and easy diagnostic test. They compared the sensitivity and specificity of this method in 2 groups of patients suspected of having CADASIL with complete scanning of mutation-causing exons of NOTCH3 (in a retrospective series of 39 patients) and with limited scanning of 4 exons that are mutation hotspots (prospective series of 42 patients). In the retrospective series, skin biopsy was positive in 21 (96%) of the 22 CADASIL patients examined and negative in all others; in the prospective series, 7 of the 42 patients had a positive skin biopsy whereas only 4 had a mutation detected by limited NOTCH3 scanning. Their immunostaining technique, based on the abnormal accumulation of NOTCH3 within small vessels, was judged to be highly sensitive (96%) and specific (100%) for the diagnosis of CADASIL.
In a study in Britain, Markus et al. (2002) found 15 different point mutations in the NOTCH3 gene in 48 families, 73% of which were in exon 4, 8% in exon 3, and 6% in each of exons 5 and 6. They suggested that on the basis of this spectrum the suggested protocol is to screen exon 4 and proceed to mutational screening of exons 3, 5, and 6 where indicated. The presence of granular osmiophilic material on skin biopsy is diagnostic but can be negative. Skin biopsy, performed in 18 cases, had a sensitivity of 45% and specificity of 100%. Anterior temporal pole involvement on MRI was considered a useful diagnostic marker.
Peters et al. (2005) identified 54 distinct mutations in the NOTCH3 gene in 120 (96%) of 125 patients with biopsy-proven CADASIL. Of the mutations, 58.3% were located in exon 4, and 85.8% were in exons 2 through 6. In 5 (4%) patients, no mutation was identified, indicating false negative results. Peters et al. (2005) suggested that cases with a high index of clinical suspicion should be investigated by skin biopsy if the result of genetic testing is negative.
In a retrospective study of 131 Finnish, Swedish, and French CADASIL patients, Tikka et al. (2009) found 100% concordance between the presence of granular osmiophilic material in skin biopsies and presence of pathogenic mutations in the NOTCH3 gene. The findings suggested that adequate skin biopsies, which must include the border zone between the deep dermis and upper subcutis that contains small arterial vessels, which contain the deposits, are a cost-effective guide for determining who should be further evaluated by molecular diagnosis. Deposits were absent in skin biopsies from 26 controls without NOTCH3 mutations.
HistorySonninen and Savontaus (1987) reported a family in which 16 patients had adult-onset multi-infarct dementia associated with occlusive cerebrovascular infarcts in the white matter, which was also generally reduced. The disorder was characterized by relapsing strokes with neuropsychiatric symptoms, affected relatively young adults of both sexes, and was transmitted in an autosomal dominant pattern. This may be the same disorder as that called familial subcortical dementia with arteriopathic leukoencephalopathy by Davous and Fallet-Bianco (1991).