Naxos Disease
A number sign (#) is used with this entry because of evidence that Naxos disease (NXD) is caused by homozygous mutation in the plakoglobin gene (JUP; 173325) on chromosome 17q21.
Another syndrome involving cardiomyopathy, woolly hair, and keratoderma (Carvajal syndrome; 605676) is caused by mutation in the desmoplakin gene (DSP; 125647). Also see 610476 for a similar disorder caused by homozygous mutation in the DSC2 gene (125645).
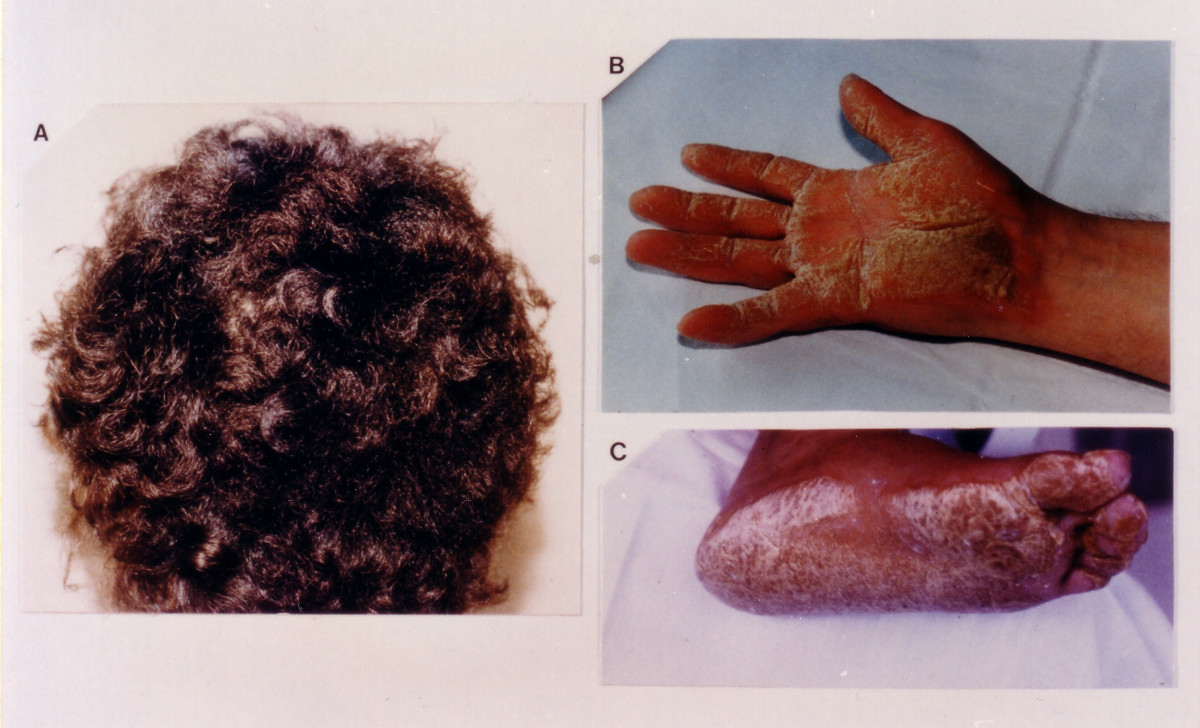
Clinical FeaturesNaxos disease is an autosomal recessive disorder that combines palmoplantar keratoderma (PPK) and other ectodermal features with cardiac disorders suggesting arrhythmogenic right ventricular dysplasia/cardiomyopathy (see ARVD/C; 107970). It was first reported in families on the Greek island of Naxos by Protonotarios et al. (1986). At that time the authors described 4 families and 9 cases. Seven of the 9 affected persons showed signs and symptoms of heart disease, including cardiomegaly, EKG abnormalities, episodes of ventricular tachycardia, and, in 1 patient, sudden death. All patients had echocardiographic evidence of enlargement of the right ventricle and a right ventricular band; in 3, the left ventricle was also affected. Protonotarios et al. (1986) pictured the scalp hair, which was described as dense, rough, and bristly, resembling steel wire. One patient was thought to have Ebstein anomaly (224700) with additional right ventricular myocardial dysplasia.
Barker et al. (1988) emphasized the association of curly hair and the cardiac abnormality with PPK. Hammill et al. (1988) reported the association of dilated cardiomyopathy with cardiac arrhythmias and ectodermal dysplasia in 3 children.
Tosti et al. (1994) described 2 members of a family who had curly hair with diffuse PPK of the Unna-Thost type (144200) associated with asymptomatic right ventricular dysfunction. A skin biopsy specimen from the plantar lesions showed only compact hyperkeratosis, hypergranulosis, and acanthosis.
Winik et al. (2009) described 2 unrelated Argentinian boys who had curly hair, PPK, and skin fragility. Both boys had trauma-induced blisters and erosions on the extremities observed from the first month of life, with later development of diffuse hyperkeratotic palmoplantar plaques that had an erythematous, well-demarcated border with erosions, blisters, and crusted lesions. Less pronounced hyperkeratosis was also present over the flexor and extensor surfaces of the joints as well as the perianal and intergluteal folds; superficially denuded areas and erosions were seen within these lesions. Hyperkeratotic and fissured plaques as well as erosive crusted lesions were observed periorally and around the ears, and the lips were usually red and cracked. Both patients had abundant curly hair, which was unusual for the prevalent ethnic type in their area of Argentina, with a normal hair shaft structure. Eyebrows were sparse in 1 patient, but both had normal long eyelashes. Nails were normal at birth but subsequently showed secondary dystrophy, and sweating was normal. Cardiovascular evaluation was normal in both children. Light microscopy of affected skin showed thickening of the epidermis with hyperkeratosis and acantholysis, and there was widening of the intercellular spaces between keratinocytes from the suprabasal layer upwards, as well as acantholysis in the superior spinous layer and granular layer. Electron microscopy confirmed widening of the intercellular spaces, most prominently in the spinous layer. Desmosomes were markedly reduced in number and poorly developed, with no clear insertion of the keratin filaments, which were clumped around the nuclei. Immunofluorescence staining of patient skin with antibodies to plakoglobin showed marked reduction in expression with diffuse peripheral membrane localization.
Cabral et al. (2010) studied 3 unrelated Argentinian boys, including the 2 previously reported by Winik et al. (2009), and a Kuwaiti sister and brother, who all presented with PPK and skin fragility as well as woolly hair, which was predominantly sparse in the Kuwaiti sibs. Transmission electron microscopy (TEM) in the Kuwaiti proband showed remarkably similar findings to those of the previously studied Argentinian patients, with loss of keratinocyte-to-keratinocyte adhesion, most prominent in the spinous layer, with aggregation of the keratin intermediate filament (IF) network. Desmosomes were hypoplastic and lacked defined keratin IF insertion. Immunohistochemical analysis of skin from the Kuwaiti and Argentinian probands showed virtually absent reactivity to antibodies raised against plakoglobin. Although all 5 children had normal resting electrocardiograms and echocardiograms, the authors noted that the oldest patient was only 14 years of age and that development of cardiomyopathy in young adulthood could not be excluded.
Pigors et al. (2011) described a female infant who was born with extensive areas of superficially eroded skin, which rapidly progressed to generalized erythema and epidermolysis with massive transcutaneous fluid loss. Complete absence of scalp hair and onycholysis were also noted. Ultrasound showed normal morphology and function of the heart and no internal abnormalities. The patient died on day 12 of life due to respiratory failure and sepsis from infection of the skin. Histopathology of the skin showed pronounced acanthosis as well as cleavage within the epidermis, with loss of upper spinous, granular, and horny layers. Basal keratinocytes were attached to the basement membrane but had little or no contact with neighboring or suprabasal cells. TEM revealed intact basement membrane and hemidesmosomes at the dermal-epidermal junction; however, desmosomes were absent on lateral aspects of basal cells and around spinous cells. Epidermal keratinocytes formed interdigitating protrusions, but no adhesion structures were recognizable. Immunofluorescence and immunoblot analysis demonstrated complete loss of plakoglobin in patient skin. The patient's parents were first cousins, and a younger brother was reported to have alopecia.
Erken et al. (2011) reported 2 men with ARVC, PPK, and alopecia from a consanguineous Turkish family. The 34-year-old proband, who had a syncopal episode at age 20, after which his only symptom was exertional dyspnea, was diagnosed with ARVC after experiencing cardiac arrest with sustained ventricular tachycardia that was terminated by cardiac defibrillation. Echocardiography revealed dilation of all heart chambers, thrombus in the right atrium, akinesia and apical aneurysm of the right ventricular free wall, and motion abnormalities of the left ventricular wall. Cardiac MRI showed dilation of both ventricles and diffuse thinning of the right ventricle. Because of refractory heart failure, the patient underwent cardiac transplantation; histopathologic examination of the explanted heart showed massive loss of myocardial muscle fibers and replacement by mature adipose tissue, particularly in the right ventricular wall. The proband's 46-year-old uncle was also diagnosed with ARVC and right heart failure, with similar findings on echocardiography; endomyocardial biopsy showed fibrotic replacement of the right ventricular free wall. Electrophysiologic studies showed easily inducible ventricular flutter, and he underwent placement of an implantable cardioverter defibrillator. Both patients had absent scalp and body hair since birth, with scant facial hair, and both also displayed mild focal PPK. Skin biopsy from the uncle's scalp showed very few hair follicles and sebaceous glands in the dermis, some of which were very small, as well as cystic dilation of the eccrine gland ducts; however, the interfollicular epidermis was normal.
InheritanceBoth Naxos disease and dilated cardiomyopathy with woolly hair and keratoderma are autosomal recessive, whereas most of the hereditary dilated cardiomyopathies are autosomal dominant (Schonberger and Seidman, 2001).
MappingIn 3 unrelated Argentinian boys with skin fragility, PPK, and woolly hair, Cabral et al. (2010) performed homozygosity mapping and identified a region of 815 homozygous SNPs on chromosome 17 that harbored the JUP gene. Pairwise identity-by-descent estimation tests of the 550,000-SNP mapping data indicated that it was highly unlikely that the 3 boys were closely related.
In a consanguineous Turkish family in which an uncle and nephew had ARVC, PPK, and alopecia, Erken et al. (2011) performed genomewide SNP genotyping and identified a 12-Mb autozygous region between SNPs rs17780388 and rs12450654 at chromosome 17q11.2-q21.32 (chr11:30,606,301-42,754,777; GRCh37), for which they obtained a maximum lod score of 3.2. Other autozygous regions detected were much shorter (less than 1 Mb).
Molecular GeneticsMcKoy et al. (2000) identified homozygosity for a 2-basepair deletion in the plakoglobin gene (173325.0001) in 19 individuals with Naxos disease. Twenty-nine clinically unaffected family members were heterozygous for the mutation; 20 unrelated individuals from Naxos and 43 autosomal dominant ARVC probands were homozygous for the normal allele.
In 3 unrelated Argentinian boys with skin fragility, PPK, and woolly hair, Cabral et al. (2010) identified homozygosity for a nonsense mutation in the JUP gene (S24X; 173325.0003). A similarly affected Kuwaiti sister and brother with predominantly sparse hair were homozygous for a splice site mutation in JUP (173325.0004). The mutations segregated with disease in each of the families and were not found in 108 control chromosomes.
In a female infant with generalized epidermolysis, alopecia, and onycholysis who died at day 12 of life due to sepsis and respiratory failure, in whom mutation in 6 genes encoding desmosomal or adherens junction components had been excluded, Pigors et al. (2011) sequenced the candidate gene JUP and identified a homozygous nonsense mutation (Q539X; 173325.0005) for which her unaffected first-cousin parents were heterozygous. Noting that the skin phenotype in this patient was more severe than that previously associated with mutations in JUP, Pigors et al. (2011) demonstrated complete lack of plakoglobin in her skin, which they stated resulted in extreme skin fragility and did not allow skin barrier formation. They also suggested that cardiac dysfunction typical of Naxos disease might have developed later in life.
In an uncle and nephew from a consanguineous Turkish family with ARVC, PPK, and alopecia mapping to chromosome 17q11.2-q21.32, Erken et al. (2011) sequenced the candidate gene JUP and identified homozygosity for a missense mutation (R265H; 173325.0006) that segregated fully with disease in the family and was not found in 192 Turkish controls.
In a review of cardiocutaneous syndromes and arrhythmogenic cardiomyopathy, Sen-Chowdhry and McKenna (2014) noted that there was evidence for a gene-dose effect in ARVC, involving probands and carrier relatives who harbor more than 1 desmosomal gene mutation, with consequent increased risk of family members developing penetrant disease. In addition, because different disease subtypes have been found to coexist within the same kindred, they suggested a role for modifier genes and/or environmental influences.
HeterogeneityDjabali et al. (2002) reported the clinical findings of members of 2 Arab families originating from villages near Jerusalem who had been diagnosed with Naxos syndrome. Affected individuals from both families had sparse eyebrows and woolly, curly, rough, light-colored scalp, axillary, and pubic hair. Skin involvement included the development of PPK in early childhood, as well as follicular keratosis on the arms, legs, back, and cheeks, lichenoid papules mainly on the lower shins, and psoriasiform keratosis. Plantar skin biopsies revealed 2 types of keratoderma, epidermolytic in family A and nonepidermolytic in family B. Hair shaft analysis demonstrated variable diameters and trauma-related abnormalities, including longitudinal and oblique fractures, tapered hairs, trichorrhexis nodosa-like lesions, pseudomonilethrix, and twisted and corkscrew-like hair without pili torti. In family A, affected individuals had various arrhythmias documented by electrocardiography, and 2 of them exhibited changes consistent with ARVC on echocardiography. Djabali et al. (2002) excluded both plakoglobin and desmoplakin as candidate genes in these families. In addition, they excluded genes encoding keratin type I (see 148020) and type II (see 148041) on chromosomes 17 and 12, respectively; desmoyokin (AHNAK; 103390) on 11q13.1; the desmocollin/desmoglein cluster on 18q12.1 (see 125643); plakophilin-1 (PKP1; 601975) on 1q32; plakophilin-2 (PKP2; 602861) on 12p13; and plakophilin-4 (PKP4; 604276) on 2q23-q31. Affected individuals from family B were later found by Ramot et al. (2014) to be homozygous for mutations in the KANK2 gene (614610); see PPKWH (616099).