Prader-Willi Syndrome
A number sign (#) is used with this entry because of evidence that Prader-Willi syndrome (PWS) is in effect a contiguous gene syndrome resulting from deletion of the paternal copies of the imprinted SNRPN gene (182279), the NDN gene (602117), and possibly other genes within the chromosome region 15q11-q13.
DescriptionPrader-Willi syndrome is characterized by diminished fetal activity, obesity, muscular hypotonia, mental retardation, short stature, hypogonadotropic hypogonadism, and small hands and feet. It can be considered to be an autosomal dominant disorder and is caused by deletion or disruption of a gene or several genes on the proximal long arm of the paternal chromosome 15 or maternal uniparental disomy 15, because the gene(s) on the maternal chromosome(s) 15 are virtually inactive through imprinting. Horsthemke and Wagstaff (2008) provided a detailed review of the mechanisms of imprinting of the Prader-Willi/Angelman syndrome (105830) region.
See also the chromosome 15q11-q13 duplication syndrome (608636), which shows overlapping clinical features.
Clinical FeaturesThe original paper by Prader et al. (1956) described the full clinical picture.
Prenatal
Mothers with prior experience of normal pregnancies almost without exception report distinctly delayed onset and reduced fetal activity during the pregnancies involving Prader-Willi children. Obstetricians often fail to detect diminished fetal activity with ultrasound investigation. When reduced fetal activity is observed, prenatal cytogenetic examination produces normal results because cytogeneticists were not instructed to look for the characteristic chromosomal changes of PWS (Schinzel, 1986). Alert clinicians should refer CVS material from pregnancies with fetuses that demonstrate poor activity for molecular diagnosis of the syndrome (see below). Other candidates for prenatal diagnosis of PWS are fetuses of pregnancies in which trisomy 15 or mosaic trisomy 15 was determined from CVS, and in which subsequent amniocyte or fetal blood examinations disclosed a normal diploid karyotype. Theoretically, one-third of trisomy 15 fetuses initially with 2 maternal chromosomes 15 and 1 paternal chromosome 15 should give rise to Prader-Willi syndrome patients exhibiting maternal uniparental disomy (Cassidy et al., 1992; Purvis-Smith et al., 1992; Hall, 1992).
Perinatal
Neonates are profoundly hypotonic, which often causes asphyxia. In addition, there is mild prenatal growth retardation with a mean birth weight of about 6 lbs (2.8 kg) at term, hyporeflexia, poor feeding due to diminished swallowing and sucking reflexes, which in many cases necessitates gavage feeding for about 3 to 4 months. Cryptorchidism occurs with hypoplastic penis and scrotum in boys and hypoplastic labiae in girls (Stephenson, 1980). Chitayat et al. (1989) commented on the normal size of hands and feet at birth and in the first year of life.
Miller et al. (1999) described 6 newborns evaluated for hypotonia who were later diagnosed with Prader-Willi syndrome. These newborns lacked the classic neonatal features of the syndrome (peculiar cry, characteristic craniofacial features, and clinical evidence of hypogonadism). The authors suggested that specific genetic testing for PWS be considered for all neonates with undiagnosed central hypotonia even in the absence of the other major features of the syndrome.
Oiglane-Shlik et al. (2006) studied 5 newborns with hypotonia, poor arousal, weak or absent cry, and no interest in food, in whom PWS was confirmed by the abnormal methylation test. All had a distinctive facial appearance, with high prominent forehead, narrow bifrontal diameter, downturned corners of the mouth, micrognathia, and dysplastic ears. Three neonates had a high-arched palate, and 4 had arachnodactyly. In the first few days of life, 4 of the 5 patients demonstrated a peculiar position of the hands, with the thumb constantly adducted over the index and middle finger. All 5 patients had transient bradycardia, thermolability, and acrocyanosis; and 3 also showed marked skin mottling, as previously reported by Chitayat et al. (1989).
Infancy and Childhood
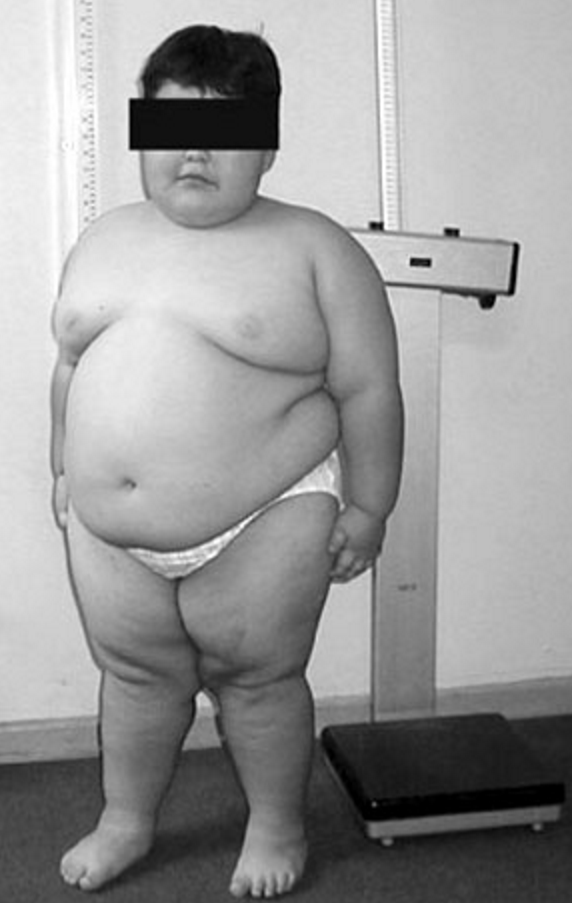
Feeding difficulties generally improve by the age of 6 months. From 12 to 18 months onward, uncontrollable hyperphagia causes major somatic as well as psychologic problems. Diminished growth is observed in the majority of infants (Butler and Meaney, 1987). Small hands with delicate and tapering fingers and small feet (acromicria) are seen in most infants and adolescents; hand and foot sizes correlate well with length, but not with age, and foot size tends to be lower than hand size. However, patients of normal height tend to have normally sized hands (Hudgins and Cassidy, 1991). The face is characterized by a narrow bifrontal diameter, almond-shaped eyes (often in mild upslanted position), strabismus, full cheeks, and diminished mimic activity due to muscular hypotonia. Plethoric obesity becomes the most striking feature. From the age of about 6 years onward, many children present scars from scratching due to itching, and later, almost all show abdominal striae.
Depigmentation relative to the familial background is a feature in about three-quarters of the patients. Butler (1989), Hittner et al. (1982), and several authors remarked that this sign is confined to cases with deletions and absent in those with maternal disomy 15. Phelan et al. (1988) presented a black female child with oculocutaneous albinism, PWS, and an interstitial deletion of 15q11.2. Patients with classic albinism (203100) have misrouting of optic fibers, with fibers from 20 degrees or more of the temporal retina crossing at the chiasm instead of projecting to the ipsilateral hemisphere. Misrouting can result in strabismus and nystagmus. Because patients with PWS have hypopigmentation and strabismus, Creel et al. (1986) studied 6 patients, selected for a history of strabismus, with pattern-onset visual evoked potentials on binocular and monocular stimulation. Of the 4 with hypopigmentation, 3 had abnormal evoked potentials indistinguishable from those recorded in albinos. The 2 with normal pigmentation had normal responses. Wiesner et al. (1987) found that 14 of 29 patients with PWS had ocular hypopigmentation. There was possible correlation between hypopigmentation and a deletion of 15q.
MacMillan et al. (1972) described 2 unrelated girls with the features of PWS who additionally showed precocious puberty. They suggested that this is a variant and that a hypothalamic disturbance is responsible for this disorder. Hall and Smith (1972) pointed out narrow bifrontal cranial diameter as a feature. Hall (1985) pointed to a possibly increased risk of leukemia in PWS.
A frequent feature generally overlooked is thick saliva at the edges of the mouth. Patients tend to be relatively insensitive to pain (including that caused by obtaining blood samples)(Prader, 1991).
Eiholzer et al. (1999) presented data on body composition and leptin (164160) levels of 13 young, still underweight children and 10 older overweight children with Prader-Willi syndrome. Both groups showed elevated skinfold standard deviation scores for body mass index and elevated body mass index-adjusted leptin levels, suggesting relatively increased body fat even in underweight children. Leptin production appeared to be intact. The authors concluded that body composition in PWS is already disturbed in infancy, long before the development of obesity.
Van Mil et al. (2001) compared body composition in 17 patients with PWS with 17 obese control patients matched for gender and bone age. In children with PWS, adiposity was associated with reduced fat-free mass, and extracellular-to-intracellular water ratio was increased. Both findings are related to growth hormone (GH; 139250) function and physical activity. Bone mineral density, especially in the limbs, tends to be reduced in patients with PWS and is related to growth hormone function.
Gunay-Aygun et al. (2001) reviewed the sensitivity of PWS diagnostic criteria and proposed revised criteria for DNA testing. From birth to 2 years any infant with hypotonia and poor suck should have DNA testing for the PWS deletion. From age 2 to 6 years any child with hypotonia and a history of poor suck and global developmental delay should have DNA testing. From 6 years to 12 years any child with history of hypotonia and poor suck, global developmental delay, and excessive eating with central obesity should be tested for PWS.
Adolescence and Adulthood
Greenswag (1987) reported on a survey of 232 adults with PWS, ranging in age from 16 to 64 years. Of 106 patients whose chromosomes were analyzed, 54 had an abnormality of chromosome 15, primarily a deletion. Physical characteristics, health problems, intelligence, psychosocial adjustment, and impact on the family were reviewed. Emotional lability, poor gross motor skills, cognitive impairment, and insatiable hunger were especially remarkable features.
Olander et al. (2000) pointed to the occurrence of 3 PWS phenotypes: patients with paternal deletions have the typical PWS phenotype; patients with maternal UPD have a slightly milder phenotype with better cognitive function; and patients with maternal UPD and mosaic trisomy 15 have the most severe phenotype with a high incidence of congenital heart disease. They described a patient with the severe phenotype with maternal isodisomy rather than the more common maternal heterodisomy. They concluded that the more severe PWS phenotype was due to trisomy 15 mosaicism rather than to homozygosity for deleterious chromosome 15 genes.
In contrast to infants, adults invariably are small compared to their family members (Butler and Meaney, 1987). Due to high caloric intake, alimentary diabetes frequently sets in during or soon after the period of puberty. Puberty itself is diminished in PWS patients of both sexes. Adolescents and young adults often require digitalization because of cardiac insufficiency; however, it has been shown that substantial weight reduction relieves the need of cardiac therapy. Any attempt to reduce food intake in these adolescents often leads to serious psychologic and behavioral problems, and in some children, the situation in their home environment becomes intolerable (Curfs et al., 1991). Patients rarely survive beyond 25 to 30 years of age, the cause of death being diabetes and cardiac failure. However, if strict weight control is achieved, both diabetes and cardiac failure are greatly reduced and survival is either not or only mildly reduced. Johnsen et al. (1967) studied 7 mentally retarded patients, aged 4 to 19 years. Studies showed that fat synthesis from acetate during fasting was 10 times greater in patients than in unaffected sibs, and that hormone-stimulated lipolysis was depressed. These workers suggested that the condition is comparable to the genetic obese-hyperglycemic mouse. Since during fasting substrate continues to be used for new fat and lipolysis is deficient, survival depends on a continuous supply of exogenous calories. The abundant fat, muscle hypotonia, and small feet and hands are exactly the opposite of the sparse fat, muscle hypertrophy, and large hands and feet in Seip syndrome (269700).
Hoybye et al. (2002) studied the clinical, genetic, endocrinologic, and metabolic findings in 10 male and 9 female adult PWS patients (mean age, 25 years). The PWS karyotype was demonstrated in 13 patients. The mean BMI was 35.6 kg/m2, and total body fat was increased. Two-thirds were biochemically hypogonadal. Fifty percent had severe GH deficiency. Four were hypertensive. One patient had heart failure and diabetes. Impaired glucose tolerance was seen in 4 patients, elevated homeostasis model assessment index in 9, and modest dyslipidemia in 7. IGF-binding protein-1 (146730) correlated negatively with insulin (176730) levels. Four patients had osteoporosis, and 11 had osteopenia. There was no significant difference between the group with the PWS karyotype and the group without the karyotype in age, BMI, waist-to-hip ratio, percent body fat, insulin values, homeostasis model assessment index, or lipid profile, except for lipoprotein(a) (152200), which was significantly higher in the group with the negative karyotype. Hoybye et al. (2002) concluded that the risk factors found predicting cardiovascular disease were secondary to GHD and emphasized the importance of evaluating treatment of GHD in adults with PWS.
Curfs et al. (1991) concluded that PWS patients score better on visual motor discrimination skills than on auditory verbal processing skills.
Wise et al. (1991) described 5 patients with PWS who experienced recurrent hyperthermia in infancy. On the basis of these patients and other reports of abnormal temperature regulation in PWS patients, particularly hypothermia with exposure to cold, they concluded that defects in temperature regulation may be a manifestation of hypothalamic dysfunction in PWS. On the other hand, Cassidy and McKillop (1991) concluded on the basis of a survey that clinically significant abnormal temperature control is not a common finding in this disorder. Similarly, Williams et al. (1994) concluded on the basis of a survey that the prevalence of febrile convulsions, fever-associated symptoms, and temperature less than 94 degrees F were not unique to PWS but can occur in any neurodevelopmentally handicapped individual and do not necessarily reflect syndrome-specific hypothalamic abnormalities.
Individuals with Prader-Willi syndrome manifest severe skin picking behavior. Bhargava et al. (1996) described 3 adolescent patients in whom an extension of this behavior to rectal picking resulted in significant lower gastrointestinal bleeding and anal rectal disease. Recognition of this behavior is important to avoid misdiagnosing inflammatory bowel disease in PWS patients.
Wharton et al. (1997) presented 6 patients with PWS with dramatic acute gastric distention. In 3 young adult women with vomiting and apparent gastroenteritis, clinical course progressed rapidly to massive gastric dilatation and gastric necrosis. One patient died of overwhelming sepsis and disseminated intravascular coagulation. In 2 children, gastric dilatation resolved spontaneously. Gastrectomy was performed in 2 cases; in 1, gastrectomy was subtotal and distal, whereas in the other, gastrectomy was combined with partial duodenectomy and pancreatectomy. All specimens showed ischemic gastroenteritis. There was diffuse mucosal infarction with multifocal transmural necrosis.
From a study of 10 African Americans with PWS, Hudgins et al. (1998) pointed out that the clinical features differ from those of white patients. Growth is less affected, hand and foot lengths usually are normal, and the facies are atypical; as a result, PWS may be underdiagnosed in this population.
Lindgren et al. (2000) studied the microstructure of eating behavior in patients with PWS and compared it with that of members of obese and normal weight control groups of the same age. PWS patients had a mean age of 10 +/- 4 years, while the control groups were 12 +/- 3 years (normal weight) and 12 +/- 4 years (obese). Subjects with PWS had a longer duration of eating rate compared with members of both obese and normal weight groups. In subjects with PWS, 56% of the eating curves were non-decelerating, compared with 10% of the normal weight group and 30% of the obese group. Lindgren et al. (2000) concluded that the eating behavior found in subjects with PWS might be due to decreased satiation rather than increased hunger.
Nagai et al. (2000) reported standard growth curves for height and weight among Japanese children with Prader-Willi syndrome. No difference in height was seen between those with and those without chromosome 15q deletion.
Cassidy et al. (1997) personally examined and studied using molecular techniques 54 individuals with PWS to determine whether there are phenotypic differences between patients with the syndrome due to deletion (present in 37) or uniparental disomy (present in 17) as the mechanism. Previously recognized increased maternal age in patients with UPD and increased frequency of hypopigmentation in those with deletion were confirmed. Although the frequency and severity of most other manifestations of PWS did not differ significantly between the 2 groups, those with UPD were less likely to have a 'typical' facial appearance. In addition, this group was less likely to show some of the minor manifestations such as skin picking, skill with jigsaw puzzles, and high pain threshold. Females and those with UPD were also older, on average.
Gunay-Aygun et al. (2001) proposed new revised criteria for DNA testing for individuals in adolescence and adulthood. Anyone with cognitive impairment (usually mild mental retardation), excessive eating with central obesity, and hypothalamic hypogonadism, and/or typical behaviors, including temper tantrums and obsessive-compulsive features, should be referred for DNA testing for PWS.
Among 25 patients with PWS aged 18 years or older, Boer et al. (2002) found that 7 (28%) had severe affective disorder with psychotic features, with a mean age of onset of 26 years. The 7 affected persons, all aged 28 years or older, included all 5 with disomies of chromosome 15, 1 with a deletion in this chromosome, and 1 with an imprinting center mutation in the same chromosome. They postulated that in PWS, an abnormal pattern of expression of a sex-specific imprinted gene on chromosome 15 is associated with psychotic illness in early adult life.
Vogels et al. (2004) detailed the psychopathologic manifestations of 6 adults with PWS and a history of psychotic episodes. Characteristics of the psychotic disorder included early and acute onset, polymorphous and shifting symptoms, psychiatric hospitalization along with precipitating stress factors, and a prodromal phase of physiologic symptoms.
To evaluate the risk of cancer in patients with PWS, Davies et al. (2003) conducted a retrospective questionnaire survey of its occurrence among patients registered with the PWS Association compared with cases in the general US population based on the SEER program. The median age of 1,024 PWS patients was 19.0 years (range, 0.1-63 years) with 2 older than age 50. The ratio of observed (8) to expected (4.8) cancers was 1.67 (p = 0.1610; 95% CI = 0.72-3.28). Three myeloid leukemias were confirmed, resulting in a ratio of observed to expected of 40.18 (p = 0.0001; 95% CI = 8.0-117). The authors speculated that a gene within the 15q11-q13 region may be involved in the biology of myeloid leukemia or that secondary manifestations of PWS, such as obesity, may be associated with an increased risk of certain cancers.
Wey et al. (2005) described a woman with features consistent with PWS due to a mosaic imprinting defect. Three independent assays revealed a reduced proportion of nonmethylated SNURF-SNRPN alleles in peripheral blood DNA. Microsatellite analysis and FISH revealed apparently normal chromosomes 15 of biparental origin. Wey et al. (2005) estimated that approximately 50% of the patient's blood cells had an imprinting defect. Apart from a rather normal facial appearance, the proband had typical features of PWS in terms of truncal obesity, small hands with tapered fingers, and small feet. Operation for strabismus had been performed. When evaluated at 21 years of age, she presented with the major signs of PWS, except for the relatively normal facial appearance. Wey et al. (2005) suggested that the patient, although presenting with atypical PWS features at birth and in infancy, had progressively acquired more pronounced PWS features during childhood and adolescence.
Sinnema et al. (2012) reported the clinical features of 12 patients over the age of 50 years with genetically confirmed PWS. Eleven patients lived in a facility, and 1 lived with his elderly mother. Half of the patients had diabetes mellitus with an average age at diagnosis of 41.6 years. Three patients had hypertension, 3 had a history of stroke, 6 had a history of fractures, 10 had foot problems, 5 had scoliosis, 9 had edema, and 6 had erysipelas. Older patients had significantly lower functioning, particularly in activities of daily living, compared to younger control patients, and the decline began around age 40. All 8 patients with maternal uniparental disomy used psychotropic medications, 7 of whom had a psychiatric disorder. None of the 4 patients with a paternal deletion had a psychiatric illness. Sinnema et al. (2012) suggested that age-associated medical problems may be exacerbated by temperature instability, decreased mobility, and high pain threshold in PWS. Overall, the constellation of features suggested premature aging in PWS, which may also result from abnormalities in sex hormone levels. Sinnema et al. (2012) noted that the life expectancy of individuals with PWS had increased in recent years, and that these individuals have specific medical and social needs as they age.
To examine survival trends and risk factors in PWS, Manzardo et al. (2018) performed a survival analysis of the Prader-Willi Syndrome Association's 40-year mortality syndrome-specific database of 486 deaths. They compared 331 deaths that occurred between the years 2000 and 2015 (Recent) with 94 deaths that occurred before 2000 (Early). The risk for all-cause mortality in PWS was 1.5 (95% CI = 1.2-1.9) times higher for the Early than for the Recent cohort, reflected in female cardiac failure (hazard ratio (HR) = 1.8; 95% CI = 1.3-2.6), pulmonary embolism (HR = 6.1; 95% CI = 1.7-22), and gastrointestinal-related (HR = 3.2; 95% CI = 1.1-7.4) causes. Accidental deaths in males increased in the Recent cohort (HR = 5.7; 95% CI = 1.2-27.1), possibly due to enhanced weight management and mobility. Risk of death from respiratory failure was unchanged.
Butler et al. (2017) reviewed causes of death in Prader-Willi syndrome using the US Prader-Willi Syndrome Association 40-year mortality survey ranging from 1973 to 2015. A total of 486 deaths were reported (263 males, 217 females, 6 unknown) between 1973 and 2015, with mean age of 29.5 +/- 16 years (2 months-67 years); 70% occurred in adulthood. Respiratory failure was the most common cause, accounting for 31% of all deaths. Males were at increased risk for presumed hyperphagia-related accidents/injuries and cardiopulmonary factors compared to females. PWS maternal disomy 15 genetic subtype showed an increased risk of death from cardiopulmonary factors compared to the deletion subtype.
Prader-Willi-like Syndrome Associated with Chromosome 6
Fryns et al. (1986) described an 8-month-old girl with a de novo 5q/6q autosomal translocation resulting in loss of the distal part of the long arm of chromosome 6 (6q23.3-qter). Clinical manifestations included abnormal facies with broad, flat nasal bridge, small nose with broad tip, bilateral epicanthus, narrow palpebral fissures, small anteverted ears, and small mouth. Other features included truncal obesity, short hands and feet, and delayed psychomotor development. Prader-Willi syndrome was suspected initially.
Villa et al. (1995) reported a 23-month-old boy with mental and psychomotor delay, minor craniofacial abnormalities, and obesity who had a de novo interstitial deletion of chromosome 6q16.2-q21. The authors noted the phenotypic similarities to Prader-Willi syndrome. In a boy with clinical features mimicking Prader-Willi syndrome, but with a normal chromosome 15, Stein et al. (1996) found a de novo interstitial deletion of 6q22.2-q23.1. The boy showed delayed development, hypotonia, seizures, hyperactive behavior, a bicuspid aortic valve with mild aortic stenosis, small hands and feet, hypogonadism, and obesity since about 4 years of age. In a 38-year-old man with moderate to severe intellectual delay, short stature, small hands and feet, small mouth, and obesity, Smith et al. (1999) found a duplication of 6q24.3-q27. The authors noted that the phenotype showed similarities to Prader-Willi syndrome.
As reviewed by Gilhuis et al. (2000), several obese patients with cytogenetic alterations in the same region of 6q had been reported; all had in common some clinical features, including obesity, hypotonia, and developmental delays, resembling Prader-Willi syndrome. However, their behavior, facial features, and additional neurologic abnormalities, as well as a lack of cytogenetic changes or imprinting mutations on chromosome 15, clearly distinguished this PWS-like phenotype from PWS patients.
Holder et al. (2000) studied a girl with early-onset obesity and a balanced translocation between 1p22.1 and 6q16.2. At 67 months of age she weighed 47.5 kg (+9.3 SD) and was 127.2 cm tall (+3.2 SD); her weight for height was +6.3 SD. The child displayed an aggressive, voracious appetite, and the obesity was thought to be due to high intake, since measured energy expenditure was normal. However, the authors noted that apart from her obesity, there were no features suggestive of PWS. Genetic analysis of the region on chromosome 6 showed that the translocation disrupted the SIM1 gene (603128). Holder et al. (2000) hypothesized that haploinsufficiency of the SIM1 gene may be responsible for the obesity. In a boy with a Prader-Willi-like phenotype, Faivre et al. (2002) identified a deletion of chromosome 6q16.1-q21. Intrauterine growth retardation, oligohydramnios, and a left clubfoot were noted during the third trimester of pregnancy. Later, generalized obesity, slightly dysmorphic facial features, small hands and feet, clumsiness, and mental retardation were observed. Molecular analysis showed that the deletion was paternal in origin and resulted in a deletion of the SIM1 gene.
Other FeaturesMiller et al. (2007) evaluated 3-dimensional brain MRI scans of 20 individuals with PWS aged 3 months to 39 years. Intracranial morphologic abnormalities included ventriculomegaly (100%), decreased volume of the parietal-occipital lobe (50%), sylvian fissure polymicrogyria (60%), and incomplete insular closure (65%).
Fan et al. (2009) found that 10 of 56 PWS patients had seizures, 9 of whom had generalized seizures attributable to PWS. The remaining patient was born with intraventricular hemorrhage and had focal epileptic discharges, which was thought to be responsible for the seizures. Eight of the 9 with PWS-related seizures had a 15q11-q13 deletion, suggesting that decreased inhibitory effects of the GABA receptor cluster in this region may play a role in epileptogenesis. Six additional patients of the 56 had paroxysmal events such as staring spells, tremor spells, and collapsing spells.
InheritanceFamilial inheritance of PWS has been described frequently. Gabilan (1962) reported a family with affected brother and sister, as well as a second in which the parents of the proband were first cousins, but his patients were not entirely typical.
Jancar (1971) reported familial incidence. Hall and Smith (1972) reported 2 affected male maternal first cousins. One was of normal stature and intelligence. DeFraites et al. (1975) observed 5 cases in 3 sibships of an inbred Louisiana Acadian kindred. Clarren and Smith (1977) reported affected sibs and affected first cousins. They found a recurrence risk of 1.6% in sibs of probands.
It is clear that chromosomal mechanisms are principally responsible for PWS and that the syndrome is caused by lack of the paternal segment 15q11.2-q12. Basically, there are 2 mechanisms by which such a loss can occur: either through deletion of just the paternal 'critical' segment or through loss of the entire paternal chromosome 15 with presence of 2 maternal homologs (uniparental maternal disomy). The opposite, i.e., maternal deletion or paternal uniparental disomy, causes another characteristic phenotype, the Angelman syndrome (AS; 105830). This indicates that both parental chromosomes are differentially imprinted, and that both are necessary for normal embryonic development.
Ming et al. (2000) described 2 cousins with Prader-Willi syndrome resulting from a submicroscopic deletion detected by fluorescence in situ hybridization. Although the karyotype was cytogenetically normal, FISH analysis showed a submicroscopic deletion of SNRPN (182279), but not the closely associated loci D15S10, D15S11, D15S63, and GABRB3 (137192). The affected female and male were offspring of brothers who carried the deletion but were clinically normal, as were also 2 paternal aunts of the probands who likewise had the deletion. The grandmother was deceased and not available for study; the grandfather did not show deletion of SNRPN. DNA methylation analysis of D15S63 was consistent with an abnormality of the imprinting center associated with PWS. Ming et al. (2000) referred to this as grandmatrilineal inheritance, which occurs when a woman with deletion of an imprinted, paternally expressed gene is at risk of having affected grandchildren through her sons. In such an instance, PWS does not become evident as long as the deletion is passed through the female line.
Occurrence of the Prader-Willi Syndrome
The vast majority of PWS cases occur sporadically. These instances include virtually all interstitial deletions, the large majority of de novo unbalanced translocations, all instances of maternal uniparental disomy with normal karyotype or with a de novo rearrangement involving chromosome 15, and almost all cases of maternal uniparental disomy with a familial rearrangement involving chromosome 15. There is no parental age effect whatsoever in the deletion cases.
For full discussion on the mode of inheritance, see Cytogenetics, below.
Recurrence Risk
Monozygotic twins are concordantly affected. However, affected sibs and cousins have repeatedly been reported, and even if a publication bias is considered, their incidence is obviously higher than the estimated incidence in the population of about 1 in 25,000 would suggest. Clarren and Smith (1977) reported affected sibs and first cousins. They found a recurrence risk of 1.6% in sibs of probands. Cassidy (1987) stated that the Prader-Willi Syndrome Association maintained a registry of PWS individuals which, as of December 1986, contained 1,595 names of affected persons in the United States and Canada. While in some of these cases the diagnosis had not been fully confirmed, in only 1 family, that reported by Lubinsky et al. (1987), was there a well-documented recurrence. Thus, it is reasonable to assume that the recurrence risk for PWS is less than 1 in 1,000 and that such recurrence is not likely to occur when a 15q interstitial deletion is identified in the proband. (As pointed out by Kennerknecht (1992), the membership of the PWS association is not limited to affected persons; 'two thirds are families and one third professionals'.)
Ledbetter et al. (1987) summarized a scientific conference on PWS. Of 195 cases studied by high resolution cytogenetic methods, deletion of chromosome 15 was detected in 116 (59.5%); other chromosome 15 abnormalities were found in 7 additional cases (3.6%). It was suggested that the recurrence risk may be as low as 1 in 1,000.
Kennerknecht (1992) used the diagnostic criteria given by Cassidy (1987) to evaluate reported cases of PWS with a view to estimating recurrence risk. Since a deletion at 15q has not been found in familial cases of PWS, except in those where del(15q) is due to familial structural chromosome rearrangement, the recurrence risk with de novo deletion should be nearly zero. In cases with familial translocation, risk estimates depend on the nature of the translocations concerned. If only 1 child is affected and the karyotype is apparently normal, Kennerknecht (1992) estimated an overall recurrence risk of 0.4%. However, if 2 or more sibs are affected, he estimated that the risk to the next sib would be 50%. If every proband were investigated cytogenetically (to ascertain unbalanced chromosome rearrangements), molecularly (with probes to detect invisible deletions and to determine the methylation pattern), and if in each instance of a paternal deletion an examination of the father was carried out, then the few instances with a high recurrence risk could be ascertained before a second child was born.
Mutagenic Factors
Strakowski and Butler (1987) found an increased incidence of paternal periconceptional employment in hydrocarbon-exposing occupations. Among 81 patients with PWS, Cassidy et al. (1989) compared the frequency of possible periconceptional occupational hydrocarbon exposure in those fathers who demonstrated a 15q deletion with the frequency in those who did not. There was no statistically significant difference between the cytogenetically different groups. In both groups, approximately half the fathers had been employed in hydrocarbon-exposing jobs. The data provided additional support for the possibility that hydrocarbon exposure is causally related to the disorder and further suggested lack of etiologic heterogeneity between the cytogenetically different groups.
CytogeneticsDeletions account for 70 to 80% of cases; the majority are interstitial deletions, many of which can be visualized by prometaphase banding examination. A minority consist of unbalanced translocations, mostly de novo, which are easily detected by routine chromosome examination. The remainder of cases are the result of maternal uniparental disomy. In most of these latter cases, cytogenetic examinations yield normal results. However, in a few cases, either balanced translocations, familial or de novo, or supernumerary small marker chromosomes, are observed.
Deletions
Butler et al. (1986) found an interstitial deletion of chromosome 15 (breakpoints q11 and q13) in 21 of 39 cases and an apparently normal karyotype in the remainder. By studying chromosome 15 heteromorphisms, the del(15q) was demonstrably paternal in origin in all cases, although both parents were normal and all deletions were de novo events. Paternal age was not increased. The exclusively paternal origin of deletions was subsequently confirmed cytogenetically and by molecular marker analysis (Magenis et al., 1990; Zori et al., 1990; Robinson et al., 1991). Examination of other series of patients by different groups resulted in the figures that two-thirds to three-fourths of PWS patients have a deletion of 15q11-q13. In less than 10%, this is due to an unbalanced translocation while the remainder have interstitial deletions.
To analyze the mechanism underlying the interstitial de novo deletions at 15q11-q13 that underlie approximately 70% of PWS cases, Carrozzo et al. (1997) genotyped 10 3-generation families of PWS-deletion patients using microsatellite markers flanking the common deletion region. By FISH and/or other molecular techniques, each patient was known to be deleted for the interval from D15S11 to GABRB3. In 5 of 7 cases, a different grandparental origin was identified for the alleles flanking the deletion, a finding significantly different from the expected frequency in light of the close position of the markers. This finding was considered highly suggestive of an unequal crossover occurring in the paternal meiosis at the breakpoint as the mechanism leading to deletion. The authors noted that asymmetric exchanges between nonsister chromatids in meiosis I have previously been demonstrated and are the basis of a number of genetic diseases. When the related sequences are part of tandemly arrayed homologous genes, nonhomologous recombination may lead to the formation of chimeric genes, such as those of Lapore hemoglobin and of the red-green pigment genes involved in abnormalities of color vision. In other instances, the deletion/duplication event may arise from the unequal recombination between repetitive elements interspersed throughout a genomic region. A misalignment between Alu-repetitive sequences has been demonstrated in duplications of the LDL-receptor gene (606945; Lehrman et al., 1987) and the HPRT gene (308000; Marcus et al., 1993).
In 2 PWS families studied by Carrozzo et al. (1997), the data were consistent with an intrachromosomal mechanism being responsible for the deletion. One of the few precedents for intrachromosomal recombination leading to human disease is provided by the recombination that occurs between the small intronless gene within intron 22 of the factor VIII gene (300841), and a copy of gene A (FSA; 305423) located 500 kb telomeric to the F8 gene, a recombination that causes severe hemophilia (306700) (Lakich et al., 1993). This rearrangement arises almost exclusively in male meioses, indicating that it is intrachromosomal. Carrozzo et al. (1997) suggested that the in-cis mechanism leading to the deletions in PWS patients may be related either to an exchange of chromosomal material between sister chromatids or to the formation of an intrachromosomal loop, either during meiosis or as a somatic event, followed by an excision of the chromosomal material lying between the recombining regions.
Deletions in PWS and AS are subdivided into 2 main groups based on their proximal breakpoints: type 1 deletions encompass the region between BP1 and BP3 (about 6 Mb) and type 2 deletions encompass the region from BP2 to BP3 (about 5.3 Mb). However, some patients have atypical deletions. Using methylation-specific multiplex ligation-dependent probe amplification to analyze the type of deletion in 88 PWS patients, Kim et al. (2012) found that 32 (36.4%) had a type 1 deletion and 49 (55.7%) had a type 2 deletion. Seven patients (8%) had atypical larger (2) or smaller (5) de novo deletions that were associated with unique phenotypic features, although there were no unifying characteristics across the group. Variable atypical clinical features in these patients included macrocephaly, microcephaly, large hands, no hypopigmentation, lack of facial gestalt, and variable cognitive impairment. Kim et al. (2012) discussed the possible role of different genes in the manifestation of different features.
In a 23-year-old woman with Prader-Willi syndrome, Bieth et al. (2015) identified a paternally transmitted 118-kb deletion of the SNORD116 gene cluster. The authors stated that this was the smallest deletion described to that time. SNORD109A and IPW (601491) were also deleted in the patient. SNORD116 expression was absent in patient cells, but present in her unaffected father's cells.
Maternal Uniparental Disomy
Nicholls et al. (1989), studying cases of PWS in which no deletion was cytologically evident using RFLP analysis, were the first to demonstrate maternal uniparental disomy (UPD) in 2 families. Two different, apparently intact, maternal chromosomes were present ('heterodisomy'), and, as with deletion cases of PWS, there was an absence of paternal genes from the 15q11-q13 segment. Robinson et al. (1991) used cytogenetic and molecular techniques to examine 37 patients with features of PWS. Clinical features in 28 of the patients were thought to fulfill diagnostic criteria for typical PWS. In 21 of these, a deletion of the 15q11.2-q12 region could be identified molecularly, including several cases in which the cytogenetic results were inconclusive. Five cases of maternal heterodisomy and 2 of isodisomy for 15q11-q13 were observed. All 9 patients who did not fulfill clinical criteria for typical PWS showed normal maternal and paternal inheritance of chromosome 15 markers; however, one of these carried a ring-15 chromosome. Thus, all typical PWS cases showed either a deletion or maternal uniparental disomy of 15q11.2-q12. As the disomy patients did not show any additional or more severe features than did the typical deletion patients, it is likely that there is only one imprinted region on chromosome 15. A significantly increased mean maternal age was found in the disomy cases, suggesting an association between increased maternal age and nondisjunction.
Mascari et al. (1992) demonstrated maternal uniparental disomy for chromosome 15 in 18 of 30 patients (60%) without a cytogenetic deletion. Furthermore, they confirmed the observation of Robinson et al. (1991) that the phenomenon was associated with advanced maternal age. In another 8 patients (27%), they identified large molecular deletions. The remaining 4 patients (13%) had evidence of normal biparental inheritance for chromosome 15; 3 of these patients were the only ones in the study which had some atypical clinical features. All told, they estimated that about 20% of cases of PWS result from maternal uniparental disomy and that, by the combined use of cytogenetic and molecular techniques, the genetic basis of PWS can be identified in at least 95% of patients.
Mitchell et al. (1996) compared 79 cases of PWS with UPD and 43 cases with deletions. Although there were no major clinical differences between the 2 classes of patients analyzed as a whole, mean maternal and paternal age were significantly higher in the UPD patients. The UPD group had a predominance of males, yet a gender bias was not seen in the deletion group. Hypopigmentation was found in 77% of the deletion group compared to only 39% of the UPD children. When the groups were analyzed by gender, females with UPD tended to be less severely affected than female deletion patients.
Mutirangura et al. (1993) demonstrated maternal heterodisomy in 10 PWS patients. Since the markers used were 13 cM from the centromere, heterodisomy indicated that maternal meiosis I nondisjunction was primarily involved in the origin of UPD. In contrast, 2 paternal disomy cases of Angelman syndrome (AS) showed isodisomy for all markers tested along the length of chromosome 15. This suggested a paternal meiosis II nondisjunction event (without crossing over) or, more likely, monosomic conception (due to maternal nondisjunction) followed by chromosome duplication. The latter mechanism would indicate that at least some instances of uniparental disomy in PWS and AS initiate as reciprocal products of maternal nondisjunction events.
Robinson et al. (1993) reported data indicating that the majority (82%) of maternal nondisjunction events leading to UPD and causing PWS involve a meiosis I error, whereas most paternal UPD Angelman syndrome cases are meiosis II or, more likely, mitotic errors. Robinson et al. (1993) made the interesting statement that the proportion of UPD cases among all PWS patients in Switzerland is higher than in the United States, which could reflect the higher mean maternal age at birth in Switzerland versus the United States.
Gold et al. (2014) studied the frequency of Prader-Willi syndrome in births conceived via assisted reproductive technology (ART). The overall incidence in those who used ART was 1.1%; the population frequency for the United States was 1.0%. However, the proportion of individuals with maternal disomy 15/imprinting defects born after ART was higher than that in the total sample, 55.6% (10 of 18) and 34.5% (431 of 1,250), respectively. As compared with naturally conceived individuals with Prader-Willi syndrome, those who were ART-conceived were more likely to have uniparental disomy and imprinting defects than deletions. This study also demonstrated no association between twinning and Prader-Willi syndrome when ART-conceived pregnancies were excluded.
Rescuing of Trisomy 15
Maternal nondisjunction does not itself directly lead to uniparental disomy but must also involve a further nondisjunction event to produce a euploid embryo. Purvis-Smith et al. (1992) have confirmed such an origin of uniparental disomy 15 resulting from 'correction' of an initial trisomy 15. Routine chorionic villus sampling performed for advanced maternal age led to detection of placental mosaicism for trisomy 15. Follow-up studies on amniotic fluid indicated a normal 46,XY karyotype with no evidence of trisomy 15, and the pregnancy continued to term. At birth, the baby was found to have PWS. Molecular analysis indicated that the mother was the sole contributor of the chromosome 15 pair in the child. Centromere/short-arm heteromorphisms were different in the 2 chromosome 15 homologs, consistent with meiosis I error. Cassidy et al. (1992) reported a similar case that supported the idea that maternal disomy can result from a 'corrected' trisomy 15 and that maternal age was a predisposing factor to nondisjunction. Thus, in any case in which trisomy or mosaic trisomy 15 has been prenatally determined through CVS examination, a molecular study should follow to exclude uniparental (paternal or) maternal disomy. This type of examination should also be considered in case of pregnancies of translocation carrier parents involving chromosome 15.
Devriendt et al. (1997) proposed partial zygotic trisomy rescue as a mechanism for mosaicism for a de novo jumping translocation of distal chromosome 15q, resulting in partial trisomy for 15q24-qter in a patient with PWS. A maternal uniparental heterodisomy for chromosome 15 was present in all cells and was responsible for the PWS phenotype. The translocated 15q segment was of paternal origin and was present as a jumping translocation, involving chromosomes 14q, 4q, and 16p. The recipient chromosomes were cytogenetically intact. Devriendt et al. (1997) reported that mental retardation was more marked in their patient than is usually observed in PWS, and proposed that this was due to partial trisomy for distal 15q.
Multiple Affected Relatives
There are several mechanisms that explain the simultaneous occurrence of affected first- and second-degree relatives in PWS families. These include translocations that give rise to maternal nondisjunction and hence effective maternal uniparental disomy for the PWS region and translocations which give rise to paternally derived deletions.
The first report of involvement of a D group translocation in PWS (later identified as a 15-15 translocation) dates back to 1963 (Buehler et al., 1963). Additional translocations were found subsequently, and after the introduction of chromosome banding it became obvious that at least one chromosome 15 was involved in all instances (Zuffardi et al., 1978; Kucerova et al., 1979; Guanti, 1980). However, the situation was further complicated by cases in which not only the proband had a translocation involving chromosome 15, but the mother and 2 normal sibs showed the seemingly identical translocation as well (Smith and Noel, 1980). In addition, there were a few cases that did not show a translocation involving chromosome 15, but had a small supernumerary chromosome, presumably an isochromosome for the short arm of an acrocentric (Fleischer-Michaelsen et al., 1979; Fujita et al., 1980; Wisniewski et al., 1980).
Smith and Noel (1980) described a family in which a Prader-Willi girl had the same balanced 4;15 translocation as her mother and other phenotypically normal family members. A second such family was observed by Smith et al. (1983). Nicholls et al. (1989) reported a similar family and demonstrated that the Prader-Willi proband had inherited the maternal translocation chromosome plus the normal maternal homolog, but no paternal 15. Therefore, having a balanced translocation