Hemifacial Microsomia
Description
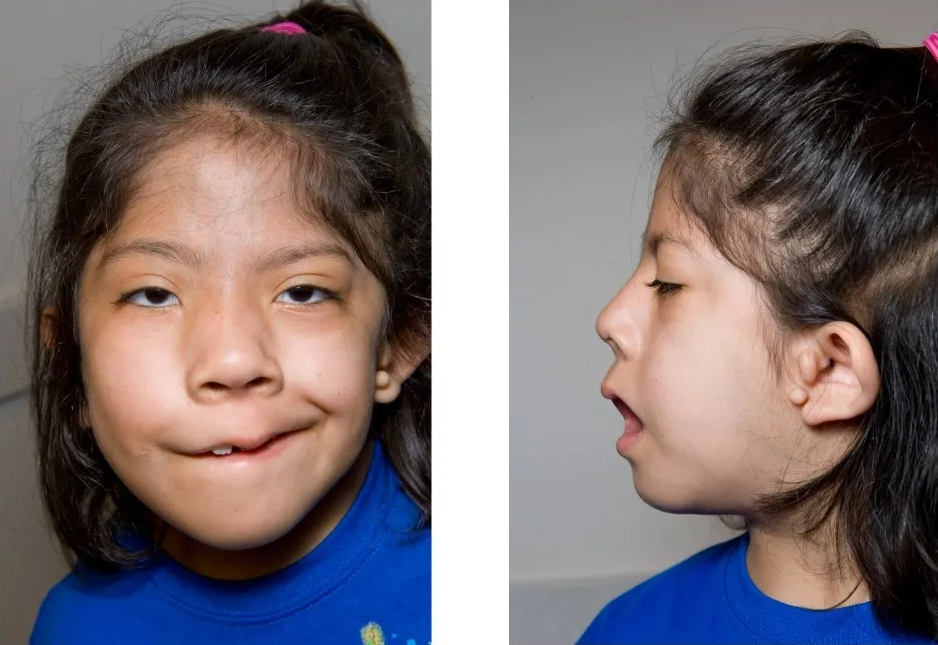
Hemifacial microsomia is a common birth defect involving the first and second branchial arch derivatives. It typically affects the external ear, middle ear, mandible and temporomandibular joint, muscles of mastication and facial muscles, and other facial soft tissues on the affected side. In some cases, other facial structures, such as the orbit, eye, nose, cranium, or neck, may be involved. Involvement is usually limited to one side, but bilateral involvement is known. In addition to craniofacial anomalies, there may be cardiac, vertebral, and central nervous system defects. The phenotype is highly variable. Most cases are sporadic, but there are rare familial cases that exhibit autosomal dominant inheritance (summary by Poole, 1989 and Hennekam et al., 2010).
See also hemifacial microsomia with radial defects (141400) and oculoauriculofrontonasal dysplasia (OAFNS; 601452), which may be part of the OAV spectrum.
Clinical FeaturesThe features of hemifacial microsomia include unilateral deformity of the external ear and small ipsilateral half of the face with epibulbar dermoid and vertebral anomalies. Coloboma of the upper eyelid is frequent. The ear deformities range from preauricular tags of cartilaginous masses, to atresia of the external auditory canal, anomalies in the size and shape of the external auricle, and even to anotia. Gorlin et al. (1963) suggested the designation oculoauriculovertebral dysplasia for this disorder.
Saraux et al. (1963) described 2 sisters with Goldenhar syndrome, born of healthy, unrelated parents. The karyotype was normal. Proto and Scullica (1966) described the condition in a father and his son and daughter. The mother was a first cousin of the father. A patient who possibly had the same condition was observed by Fraser (1967) to have acroosteolysis of the terminal phalanges. Krause (1970) described affected brother and sister. The proband had a hemangioma of the scalp. Rollnick and Kaye (1983) studied the families of 97 probands. Of 433 first-degree relatives, 35 (8%) had the same or a similar anomaly. Of 176 sibs, 11 (6%) were 'affected.' The most frequent anomaly was a mild ear malformation such as preauricular nodule or tag. Multifactorial determination was proposed. Rollnick et al. (1987) reviewed phenotypic characteristics of 294 patients.
Morrison et al. (1992) reviewed the cardiovascular anomalies associated with OAV dysplasia.
Sutphen et al. (1995) found that 3 of 60 patients with the oculoauriculovertebral spectrum (OAVS) had tracheoesophageal fistula, with or without esophageal atresia.
Stoll et al. (1998) described a mother and 2 of her children who had the oculoauriculovertebral spectrum. The mother had only auricular anomalies for which she had had plastic and reconstructive surgery. Her first child, a girl, had a bilateral cleft lip and palate, a coloboma of the upper eyelid, facial asymmetry, and posteriorly angulated ears. This child also had bilateral vesicoureteral reflux. During the second pregnancy, her fetal ultrasonographic examination performed at 18 weeks' gestation showed a cleft lip and palate. At 31 weeks' gestation, clubfeet, hypoplasia of the left ear, hypoplasia of the left maxillary and mandibular arches, and left microphthalmia were evident. Examination of the male fetus after termination of the pregnancy confirmed the findings of ultrasonography and demonstrated vertebral anomalies.
Van Meter and Weaver (1996) reported 2 infants (a male and a female) with significant overlap of symptoms of oculoauriculovertebral spectrum and CHARGE association (214800). In addition, both infants had plagiocephaly and torticollis, and the boy had cleft lip, heminostril, and tracheoesophageal fistula. The authors suggested that deficiency in migration of neural crest cells, deficiency of mesodermal formation, or defective interaction between neural crest cells and mesoderm may explain the pathogenesis of these defects of blastogenesis.
Nijhawan et al. (2002) reported 7 children with Goldenhar syndrome who were found to have dysplastic, ectopic, or bilobed caruncles, a small body at the medial angle of the eye that contains modified sebaceous and sweat glands. Four of the children had a nasal sinus or tag and 1 had a nasal-ocular cleft, suggesting an abnormality in the developmental process that is common to the nose and caruncle.
By study of a series of 30 cases (26 previously reported) and by analysis of a congenital birth defects registry in Spain, Wang et al. (2002) found that infants of diabetic mothers were at increased risk for a diagnosis of OAVS. Analysis of the registry data gave an odds ratio of OAVS of 2.28 (95% CI, 1.03-4.82; p = 0.03) and 1.50 (95% CI, 0.08-9.99; p = 0.49) in maternal gestational diabetes and preconceptually diagnosed type I or II maternal diabetes, respectively. Wang et al. (2002) hypothesized that poorly controlled maternal diabetes interferes with neural crest cell migration.
Derbent et al. (2003) described a child with del22q11 who had a phenotypic appearance similar to that seen in OAVS. He had left microtia with atresia of the external meatus, left mandibular hypoplasia, and peripheral facial nerve paralysis. Growth parameters were below the 5th percentile, but intelligence and ophthalmologic examination were normal. Cardiac findings included situs inversus dextrocardia, double outlet right ventricle, pulmonic stenosis, and ventricular septal defect. Radiographs showed platybasia, complete fusion of the C2-C3, and posterior fusion of the T1-T2 vertebrae.
Beck et al. (2005) described 2 unrelated girls with OAVS and ocular colobomas. One girl had bilateral iris colobomas extending into the retina and a small left optic nerve coloboma; her mother had features consistent with OAVS and a segmental area of heterochromia of her right iris. The second girl had a large left inferior chorioretinal coloboma involving her optic nerve and macula as well as a left colobomatous cyst; her sister and father had features consistent with OAVS, although neither had ocular colobomas.
Touliatou et al. (2006) reported detailed clinical features of 17 Greek patients with Goldenhar syndrome, including a pair of affected monozygotic twins. The most consistent findings were auricular defects (94%), followed by facial anomalies (76%), ocular anomalies (65%), and conductive hearing loss (76%). Most features were unilateral (70%); mandibular hypoplasia was ipsilateral to the dysplastic ear in 9 of 10 patients. Facial nerve paralysis and mental retardation were also observed.
Stromland et al. (2007) performed a multidisciplinary study of 18 Swedish patients with Goldenhar syndrome and found that the most frequent systemic malformations involved the ears (100%), eyes (72%), vertebrae (76%), cerebrum (50%), and heart (33%). Functional defects consisted of hearing impairment (83%; including conductive loss, sensorineural, or combined), visual impairment (28%), both visual and hearing impairment (28%), difficulties in feeding/eating (50%), speech impairment (53%), and mental retardation (39%). Two of 17 patients evaluated for autistic spectrum disorder had severe autistic symptoms (11%).
Yusufoglu et al. (2008) reported an 8-year-old boy with facial, auricular, and vertebral anomalies consistent with Goldenhar syndrome, who had a parachute mitral valve and was also found to have growth hormone deficiency. The authors noted that 1 other patient with Goldenhar syndrome had been reported with growth hormone deficiency (Nakagawa et al., 1993), but stated that it was not clear whether this was an association by chance or a rare finding in the syndrome.
Amalnath et al. (2008) reported 3 brothers with OAVS manifest as bilateral lower facial palsies, bilateral microtia with aural atresia, and kyphoscoliosis. Radiographs showed fused cervical vertebrae and spina bifida occulta of multiple lumbar vertebrae in all 3 sibs. CT scan of the temporal bone revealed temporal bone dysplasia, absent styloid process, and intact ear ossicles. There were no cardiac or renal anomalies. One of the boys developed a B cell non-Hodgkin lymphoma at age 13 years. Inheritance was consistent with an autosomal recessive or X-linked pattern.
Digilio et al. (2008) analyzed the frequency and anatomic characteristics of congenital heart defects (CHDs) in a series of 87 patients with OAVS who were examined during a 17-year period at a single center. CHDs were diagnosed in 28 (32%) of the patients. The most common CHDs were membranous or muscular ventricular septal defect (6 patients) and tetralogy of Fallot, either classic or with pulmonary atresia (6 patients). Three patients had atrial septal defect. In addition, anomalous pulmonary venous return was diagnosed in 4 patients, which was partial in 3 and total in 1. Although there is a greater frequency of male patients with OAVS, there was no sex preponderance among OAVS patients with CHDs.
Slavotinek and Vargervik (2011) reported a 10-year-old Hispanic boy who had features of OAVS, including facial asymmetry, lipodermoids, smaller right ear with preauricular tags and a cheek tag, small jaw with defective oral commissure, and vertebral anomalies, but who also displayed several atypical findings, including an imperforate anus, rib fusions, and scoliosis requiring a brace. He was born of a twin pregnancy with dichorionic, diamniotic membranes; his twin brother had developmental delay, unilateral mild to moderate conductive hearing loss, mild hypotonia, and bilateral fifth finger clinodactyly. Chromosome microarray showed no diagnostic cytogenetic aberrations, and screening of the SALL1 gene was negative in the proband.
Reviews
Beleza-Meireles et al. (2014) reviewed 158 published cases of OAVS, noting the lack of consensus regarding the minimum diagnostic criteria due to variable expressivity, with a phenotypic spectrum ranging from subtle facial asymmetry with a small skin tag in front of an otherwise normal-appearing ear to a complex phenotype involving multiple congenital anomalies. Nearly all OAVS patients exhibit some degree of hemifacial microsomia, resulting from maxillary and/or mandibular hypoplasia. External ear anomalies are also very common, and most affected individuals have some degree of hearing loss. In addition, a variety of ocular anomalies may be observed, and congenital heart defects are not uncommon.
DiagnosisPrenatal Diagnosis
Castori et al. (2006) presented a case of OAVS diagnosed prenatally with multiple congenital anomalies and reviewed the prenatal ultrasound findings of 20 previously reported cases. Gestational age at diagnosis ranged from 14 to 35 weeks, and almost half of the cases were associated with either poly- or oligohydramnios. Facial structures were involved in 52% and included microphthalmia, ear anomalies, facial asymmetry, and facial cleft. Central nervous system defects occurred in 47% and included hydrocephalus, occipital encephalocele, and cerebellar hypoplasia. Congenital heart defects, primarily atrioventricular septal defects, occurred in 19%. Additional findings included hydroureteronephrosis, radial aplasia, and lung and kidney agenesis.
InheritanceAlthough most cases are sporadic and a few families consistent with autosomal recessive inheritance have been reported, other families clearly support autosomal dominant inheritance. For example, Regenbogen et al. (1982) described a kindred with 9 affected persons in 3 generations and 3 instances of male-to-male transmission. The authors found at least 4 other reported instances of presumed autosomal dominant inheritance. Summitt (1969) described a kindred with many affected persons in an autosomal dominant pattern including male-to-male transmission. Notable variability in the clinical picture was described. Regenbogen et al. (1982) suggested that eye involvement may be less marked in the dominant form. Godel et al. (1982) described an Oriental Jewish family with 9 affected persons in 3 generations, including 3 instances of male-to-male transmission.
Kaye et al. (1992) analyzed the families of 74 probands. As the basis for segregation analysis, criteria used in evaluating relatives as affected were outlined. Relatives were examined to identify ear malformations, mandibular anomalies, and other craniofacial abnormalities. They concluded that the hypothesis of no genetic transmission could be rejected; the evidence favored autosomal dominant inheritance, whereas recessive and polygenic models were not distinguishable.
Tasse et al. (2007) reported a mother and 2 daughters with OAVS and reviewed 8 previously published familial cases. The authors found that patients with autosomal dominant inheritance of OAVS are more often bilaterally affected than sporadic patients, and that hearing loss, absent or narrow external auditory canal, anomalies of the mouth, and epibulbar dermoids appear to occur less frequently in patients with autosomal dominant inheritance than in sporadic patients.
Twin Studies
Setzer et al. (1981) reported 2 pairs of discordant monozygotic twins and an instance of affected mother and son and mother's sister. They suggested genetic heterogeneity. Discordant monozygotic twins were reported by Burck (1983), who gave an extensive review of the literature. Connor and Fernandez (1984), who considered hemifacial microsomia to be identical with Goldenhar syndrome, reported discordant monozygotic twins. They assembled from the literature 14 monozygotic twin pairs of whom only 2 were concordant. Such would be consistent with a somatic mutation as the origin.
Boles et al. (1987) observed a male twin pair discordant for epibulbar dermoids, auricular appendages, malformed auricles, and hemifacial microsomia. The twins were dichorionic but apparently monozygotic by blood grouping, cytogenetic, and dermatoglyphic criteria. Some 20 twin pairs in all have been reported in which at least 1 member exhibited the features of Goldenhar syndrome. All of the 5 monozygotic twin pairs for which placental information was available have been discordant and 2 of these had dichorionic membranes. The failure of discordant monozygotic twins to be limited to monochorionic pairs argued against the idea that developmental abnormalities arising from placental vascular anastomoses could explain the discordant expression.
PathogenesisSoltan and Holmes (1986) suggested a link between genetic causes and vascular disruption in Goldenhar anomaly. They described 5 close relatives with different malformations usually attributed to vascular accidents, including 1 with congenital microtia with preauricular appendages, a possible variant of the Goldenhar anomaly.
Jongbloet (1987) suggested that sporadically occurring Goldenhar syndrome is the result of 'overripeness ovopathy' and that this and overlapping anomalies 'should be considered to be just casualties in the broad continuum of reproductive wastage seen in high risk conceptions.' He cited an example of Goldenhar syndrome in one of triplets derived from in vitro fertilization.
Ryan et al. (1988) observed the Goldenhar anomaly in both of presumably monozygotic twins, but severity was strikingly different. They referred to work suggesting that in some cases the Goldenhar anomaly results from fetal hemorrhage in the region of the first and second branchial arches at the time when the blood supply of these arches switches from the stapedial artery to the external carotid artery.
Using a statistical methodology, Kallen et al. (2004) attempted to identify a group of probable cases of OAV dysplasia and to investigate the possible relationships between different patterns of congenital malformations. Among 5,260 infants with multiple malformations collected from 4 large registers of congenital malformations, they identified 312 probable OAV cases. With the same technique, they had previously defined epidemiologic delineations of 3 other well-known nonrandom associations of congenital malformations: CHARGE, VATER (192350), and OEIS (258040). They found convincing relationships between OAV and VATER or CHARGE, but none between OAV and OEIS or between the 3 malformation complexes CHARGE, VATER, and OEIS. Kallen et al. (2004) suggested that the connection between OAV and CHARGE could be related to a common pathogenetic mechanism, namely, disturbed neural crest development.
Among 72 sporadic patients with OAVS, Wieczorek et al. (2007) found that 4 of 10 affected twin pairs were conceived by assisted reproductive technology. Among naturally conceived patients with OAVS, there were a higher frequency of twins (7.46%) compared to the general population (1.4%). In a prospective follow-up of 3,372 fetuses conceived by intracytoplasmic sperm injection, the authors found 3 cases of OAVS, which was significantly greater than in the general population. Wieczorek et al. (2007) found that the incidence of some reproductive abnormalities is increased in parents of patients with OAVS, and suggested that assisted reproductive technologies are associated with increased risk of OAVS. The findings were compatible with the concept of 'overripeness ovopathy' as discussed by Jongbloet (1987).
MappingKelberman et al. (2001) performed a genomewide search for linkage in 2 families with features of HFM. In 1 family, the data were highly suggestive of linkage to a region of approximately 10.7 cM on chromosome 14q32, with a maximum multipoint lod score of 3.00 between microsatellite markers D14S987 and D14S65. Linkage to this region was excluded in the second family, suggesting genetic heterogeneity.
CytogeneticsKobrynski et al. (1993) reviewed 13 chromosomal aberrations that had been described in association with the FAV sequence and added the case of an infant girl born with complete trisomy 22 and features of this disorder: left hemifacial microsomia, ear anomaly, and limbal and epibulbar complex choristoma.
Choong et al. (2003) reported a male infant, born of nonconsanguineous parents, who had clinical features of Goldenhar syndrome and the cri-du-chat syndrome (123450). At birth, he was noted to have dysmorphic facial features, including bilateral preauricular tags, rotated ears, epicanthal folds, a left epibulbar lipodermoid, and an accessory left nipple. He also had hearing loss and feeding difficulties due to esophageal atresia with tracheoesophageal fistula, and horseshoe kidney. In addition, he had a high-pitched, cat-like cry, characteristic of cri-du-chat syndrome. Cytogenetic analysis detected a terminal deletion of chromosome 5p14, consistent with the cri-du-chat locus. The association of Goldenhar syndrome and cri-du-chat syndrome in this patient suggested that the chromosome 5p14 locus may harbor a gene implicated with Goldenhar syndrome.
Josifova et al. (2004) reported 2 sibs with features of Goldenhar syndrome associated with an unbalanced translocation t(5;8)(p15.31;p23.1), resulting in monosomy for 5pter-p15.31 and trisomy for 8pter-p23.2. The unaffected father was a carrier of the balanced rearrangement 46,XY t(5;8)(p15.31;8p23.1).
Descartes (2006) reported a girl with Goldenhar syndrome associated with a 5pter-p15.33 deletion. She had multiple congenital anomalies of the face and upper extremity, including frontal bossing, right and left ear tags, right ear pit, mild microretrognathia, and right hand anomalies. Descartes (2006) noted that Ladekarl (1968) had reported a patient with features of Goldenhar syndrome and cri-du-chat syndrome associated with a 5q deletion. However, the deletion in the patient reported by Descartes (2006) did not include the refined cri-du-chat locus at 5p15.3-p15.2.
Ala-Mello et al. (2008) reported a girl with features of Goldenhar syndrome and hematologic abnormalities associated with a complex translocation resulting in a deletion of 5pter-p15.3, deletion of 21q22.3-qter, and duplication of 21q22.11-q22.12. She had asymmetric macrosomia, cleft of the upper left lip, epibulbar dermoids, and preauricular tags. She had poor speech development and hearing loss. She also developed myelodysplasia necessitating bone marrow transplantation. Ala-Mello et al. (2008) postulated that her hematologic issues were related to the anomalies on chromosome 21.
Molecular GeneticsKeegan et al. (2001) performed a retrospective analysis of 8 patients with HFM-expanded spectrum and anal anomalies to determine whether this subset had Townes-Brocks syndrome (TBS; 107480). Two patients had major phenotypic findings of TBS. Sequencing of the SALL1 gene (602218) in 4 of the 8 patients revealed 1 with a C-to-T transition resulting in the nonsense mutation arg276 to ter (602218.0003) at a mutation hotspot. Keegan et al. (2001) suggested that patients with overlapping features of both Townes-Brock syndrome and hemifacial microsomia-expanded spectrum should be screened for SALL1 mutations.
Kosaki et al. (2007) identified heterozygosity for a mutation in the SALL1 gene (L419X; 602218.0010) in 2 sisters, 1 with a Townes-Brocks syndrome phenotype and the other exhibiting features of Goldenhar syndrome, including epibulbar dermoid, bilateral microtia with atretic ear canals, mandibular hypoplasia, right preaxial polydactyly, anteriorly placed anus, and hypoplastic kidneys. Their mother, who had dysplastic external ears but was otherwise normal, also carried the mutation. The authors noted that Johnson et al. (1996) had previously reported an affected family member with a Goldenhar syndrome-like phenotype in a 3-generation TBS family.
In a patient with OAVS, multiple exostoses, and a translocation t(4;8)(p15.3;q24.1), who was previously reported by Ludecke et al. (1999), Fischer et al. (2006) found that the chromosome 4 breakpoint was 76.4 kb distal to the BAPX1 gene (602183). Fischer et al. (2006) sequenced the BAPX1 gene in 105 patients with OAVS and found no mutations. However, quantitative analysis of allelic expression in fibroblasts from 12 patients and 9 controls who were heterozygous for expressed polymorphisms revealed strong allelic expression imbalance in 5 of 12 patients that was not seen in controls (Fisher exact test, p = 0.038). There was no association between a particular sequence variant and its relative transcript level. Prolonged cell culture or treatment with a histone deacetylase inhibitor led to reactivation of the downregulated allele. Fischer et al. (2006) suggested that epigenetic dysregulation of BAPX1 involving histone acetylation-dependent allelic expression imbalance predisposes to OAVS.
Su et al. (2007) analyzed the TCOF1 gene in 3 patients who had features consistent with partial OAVS, 1 patient described as having typical OAVS, and 1 patient with Treacher-Collins syndrome (TCS; 154500). They identified a gln741-to-ter (Q741X) mutation in the TCS patient. In the patient with typical OAVS, they identified an ala362-to-thr (A362T) mutation in exon 9, as well as 2 silent mutations in exons 10 and 23 (G453G and K1263K, respectively). All 5 patients carried 8 previously reported TCOF1 polymorphisms, as well as 4 novel polymorphisms that were also detected in 51 Taiwanese controls. Su et al. (2007) suggested that more patients, including familial cases, needed to be studied to confirm the genotype/phenotype correlation in OAVS.
From a French cohort of 169 OAVS patients, Lopez et al. (2016) performed whole-exome sequencing in 5 sporadic patients and identified 1 female patient with a de novo nonsense mutation (c.25C-T, R9X, NM_004535.2) in the MYT1 gene (600379). The patient exhibited right hemifacial microsomia, right epibulbar dermoid, preauricular tags and small dysplastic right pinna, moderate conductive deafness on the right with stenosis of external auditory canal and ossicular malformation of the incus and malleus, hemivertebrae and fused dorsal vertebrae, ventricular septal defect, and atypical epilepsy without loss of consciousness. Cohort screening for additional MYT1 mutations revealed 1 male OAVS patient with a missense mutation (c.314C-T, S105L) in MYT1, inherited from his unaffected father. The proband showed hemifacial microsomia, preauricular tag and dysplastic left pinna, bilateral conductive hearing loss, and lumbar dysraphism with lipoma of filum terminale without vertebral malformation. His father had progressive bilateral sensorineural hearing loss diagnosed at age 25 years, and thoracolumbar scoliosis without vertebral malformation. The S105L mutation was reported once in the ExAC database (1/120,614) and once in the Catalog of Somatic Mutations in Cancer (COSMIC) database, in association with a tumor of the large intestine. Lopez et al. (2016) concluded that MYT1 was a candidate gene for OAVS and that additional patients with mutations in MYT1 were required to establish the causality of the gene.
Berenguer et al. (2017) screened 57 OAVS patients originating from Brazil for mutations in the MYT1 gene, and identified a novel de novo missense variant (c.323C-T, S108L, NM_004535.2) in a patient with a severe phenotype. The patient was a 17-year-old female with right anotia and left microtia with left preauricular tag and cervical pits. She had conductive hearing loss on the right and mixed hearing loss on the left, as well as hemifacial microsomia, eye abnormality, abnormal orbital position, and micrognathia. Atrial septal defect (ASD) and VSD were present, and spinal x-rays revealed cervical vertebral fusion with reduced spaces from C5 to C7 and from L3 to S1. In vitro functional studies of the mutation found that MYT1 overexpression downregulated all retinoic acid receptor genes (RARA, 180240; RARB, 180220; and RARG, 180190), which are involved in retinoic acid-mediated transcription, whereas no effect was observed on expression of CYP26A1 (602239), the major enzyme involved in retinoic acid degradation.
Exclusion Studies
Based on mapping, mouse expression, and phenotype data, Kelberman et al. (2001) considered the goosecoid gene (GSC; 138890) to be an excellent candidate gene for HFM. They searched the GSC coding region for mutations in 2 families segregating HFM and in 120 sporadic cases; none was found. They excluded gross rearrangements of the gene by Southern blot analysis.
In 4 patients with Goldenhar syndrome, Splendore et al. (2002) found no mutation in the TCOF1 gene (606847), which is mutant in Treacher Collins syndrome-1 (154500).
Goodin et al. (2009) analyzed the EYA1 (601653) and SALL1 (602218) genes in 3 families segregating autosomal dominant OAVS, but found no changes. The authors suggested that, at least for these 3 families, autosomal dominant OAVS is genetically distinct from the clinically similar syndromes caused by mutation in those 2 genes, branchiootorenal syndrome (113650) and Townes-Brocks syndrome (107480), respectively.
Population GeneticsHennekam et al. (2010) estimated the incidence of HFM to be 1 in 5,600.
Morrison et al. (1992) estimated a minimum prevalence rate of OAV dysplasia of 1 in 45,000 in Northern Ireland.
Animal ModelNaora et al. (1994) described a transgenic mouse line that carries an autosomal dominant insertion mutation that results in hemifacial microsomia, including microtia and/or abnormal bite. The locus, designated Hfm for hemifacial microsomia-associated locus, was mapped to mouse chromosome 10 by in situ hybridization. By using sequences flanking the insert, the preintegration region was isolated. Analysis demonstrated that a deletion of at least 23 kb had occurred in association with the transgene integration. Homozygosity may result in prenatal lethality. They interpreted the results as suggesting that the Hfm gene is necessary for prenatal development.