Craniosynostosis 1
A number sign (#) is used with this entry because of evidence that isolated craniosynostosis-1 (CRS1) is caused by heterozygous mutation in the TWIST1 gene (601622) on chromosome 7p21.
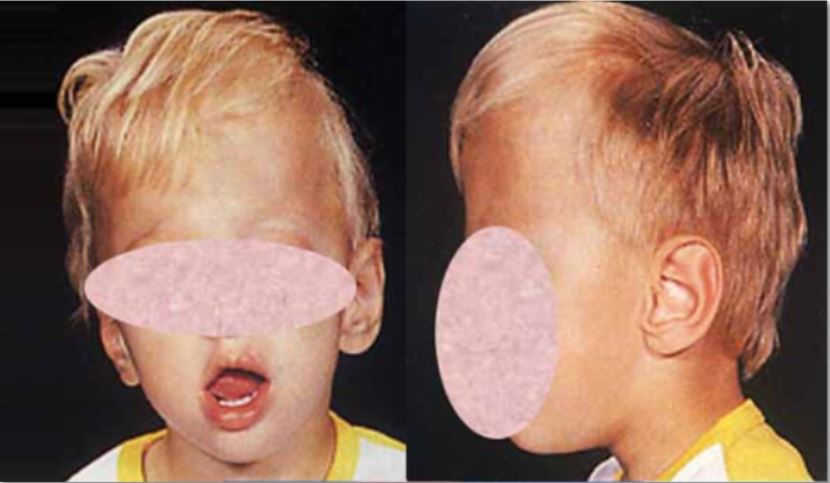
DescriptionCraniosynostosis is a primary abnormality of skull growth involving premature fusion of the cranial sutures such that the growth velocity of the skull often cannot match that of the developing brain. This produces skull deformity and, in some cases, raises intracranial pressure, which must be treated promptly to avoid permanent neurodevelopmental disability (summary by Fitzpatrick, 2013). Mutation in the TWIST1 has been found to cause coronal and sagittal forms of craniosynostosis.
Genetic Heterogeneity of Craniosynostosis
Craniosynostosis-2 (CRS2; 604757) is caused by mutation in the MSX2 gene (123101) on chromosome 5q35. Craniosynostosis-3 (CRS3; 615314) is caused by mutation in the TCF12 gene (600480) on chromosome 15q21. Craniosynostosis-4 (CRS4; 600775) is caused by mutation in the ERF gene (611888) on chromosome 19q13. Susceptibility to craniosynostosis-5 (CRS5; 615529) is conferred by variation in the ALX4 gene (605420) on chromosome 11p11. Craniosynostosis-6 (CRS6; 616602) is caused by mutation in the ZIC1 gene (600470) on chromosome 3q24. Susceptibility to craniosynostosis-7 (CRS7; 617439) is conferred by variation in the SMAD6 gene (602931) on chromosome 15q22.
Clinical FeaturesGordon (1959) found multiple cases in 5 of 9 South African families studied in detail. In 4 families, multiple sibs were involved. In the fifth, the mother of an affected child was also affected.
Bell et al. (1961) described the same condition under the designation 'scaphocephaly' in 2 families. In 1 family, 6 persons in 3 generations were said to be affected with male-to-male transmission and in another family 2 children of an unaffected woman, each by a different father, were affected.
Murphy (1953) observed craniostenosis in father and son. Nance and Engel (1967) described a family in which the mother had marked dolichocephaly and 2 sons had severe craniostenosis with premature closure of sutures and a 'beaten metal' appearance of the calvaria by x-ray. The normal father and the 2 sons had a deletion of the short arm of one G chromosome which has been found as a normal variation in some families ('Christchurch chromosome') and was found by these workers in a patient with pycnodysostosis (265800) in which failure of closure of cranial sutures is a feature. Anderson and Geiger (1965) observed an infant with left coronal synostosis and father with sagittal synostosis. Sheldon (1931) reported 5 cases of oxycephaly in 3 generations. Intelligence was normal. The membrane bones of the skull showed a 'beaten copper' appearance by x-ray.
In a large study of 519 cases of craniostenosis, Shillito and Matson (1968) encountered 9 families, in each of which 2 sibs were affected. In 1 family the sibs were identical twins. Four pairs had synostosis of one or more coronal sutures. Familial involvement was highest in cases with coronal synostosis, particularly bilateral coronal involvement. Successive generations were especially often affected in cases of multiple or total synostosis.
Kosnik et al. (1975) reported 3 families, each with multiple cases of coronal craniosynostosis. Craniosynostosis is a very heterogeneous trait, with or without associated malformation. Pillar et al. (1995) described 2 pairs of sibs with holocalvarial craniosynostosis with normal psychomotor development and minimal associated dysmorphic features, which included telecanthus, exophthalmos, mild micrognathia, and clinodactyly, but normal psychomotor development. There was a boy and a girl in each sibship. The fathers of each sibship were first cousins, but the mothers were not known to be related. Although the fathers appeared clinically normal, x-ray examination demonstrated mild generalized hyperostosis and fused sutures. On the strength of these radiographic findings in the fathers, the authors suggested autosomal dominant transmission of a self-limited disorder that may not require surgical intervention.
InheritanceCraniosynostosis-1 is an autosomal dominant trait (Seto et al., 2007).
Hunter and Rudd (1976) did a systematic study of 214 cases of sagittal synostosis without involvement of the coronal sutures. Although a few familial cases were observed, they concluded the familial incidence was only that to be expected of a multifactorial trait, i.e., the frequency in first-degree relatives was close to the square root of the population incidence as predicted by Edwards (1960).
From a series of 1,408 patients with craniosynostosis hospitalized between 1976 and 1994, Lajeunie et al. (1996) identified 561 probands with isolated scaphocephaly (sagittal craniosynostosis). Genetic analysis was performed in 366 families. The male:female ratio was 3.5:1. A proven history of scaphocephaly was found in 22 families (6%). Eleven families with normal parents had more than one affected child. In 4 families, the father of the proband had scaphocephaly. In 7 families, the parents were nonmanifesting carriers. Segregation analysis on 253 families indicated that scaphocephaly is transmitted as a dominant disorder with 38% penetrance and 72% sporadic cases. No maternal or paternal age effect was found. The frequency of twinning among 373 probands was 4.8% with only 1 concordance for sagittal synostosis in a monozygotic twin pair, supporting the hypothesis of intrauterine head constraint in the genesis of craniosynostosis.
Although most reports have suggested dominant inheritance of isolated craniosynostosis, Duguid (1929) found it in 4 sibs. Gillot et al. (1960) reported craniosynostosis in a brother and sister whose parents and 3 sibs were unaffected. Gaudier et al. (1967) reviewed the subject and reported a series of cases which included an affected brother and sister. Armendares (1970) also presented evidence supporting recessive inheritance. He pointed out that the particular deformity of the skull is dependent on which sutures close first, and the exact type of skull deformity resulting from the primary process of premature closure of the sutures varies not only between families but even within families.
Population GeneticsFitzpatrick (2013) stated that craniosynostosis affects 1 in 2,200 individuals.
MappingDeletion in the short arm of chromosome 7 has been associated with craniosynostosis (e.g., McPherson et al., 1976; Dhadial and Smith, 1979). Motegi et al. (1985) found a small deletion of 7p in a 5-month-old boy with craniosynostosis and many other anomalies. His karyotype was 46,XY,del(7)(p15.3p21.3). They found 5 previously reported cases of 7p deletion associated with craniosynostosis. They concluded that the determinant of craniosynostosis lies in the midportion of 7p21, i.e., at 7p21.2 or proximal 7p21.3. Garcia-Esquivel et al. (1986) described a 4-year-old boy with typical 7p2-monosomy syndrome, including craniosynostosis, due to a de novo interstitial deletion. They concluded that the critical deletion is band 7p21 (probably only subband p21.1). Schomig-Spingler et al. (1986) described craniosynostosis and other malformations in association with a deletion of 7pter-p21. In a patient with an unbalanced translocation resulting in trisomy for the segment 7pter-p15, Caiulo et al. (1989) found abnormalities in the skull consistent with the location of genes involved in skull development in band 7p21; the patient showed scaphocephaly, frontal bossing, and diastasis of cranial sutures at birth.
Molecular GeneticsSeto et al. (2007) performed mutation analysis in 164 infants with isolated single-suture craniosynostosis and identified novel heterozygous missense mutations in the TWIST1 gene in 2 patients, 1 with coronal (601622.0013) and 1 with sagittal (601622.0014) synostosis. Neither patient had facial anomalies or 2-3 syndactyly, although one had prominent horizontal crura of the ears and the other had small square-shaped ears, a feature shared by his otherwise unaffected father who also carried the mutation.
Associations Pending Confirmation
By exome sequencing in 191 probands with nonsyndromic midline craniosynostosis, Timberlake et al. (2016) identified heterozygous loss-of-function mutations in the SPRY1 (602465) and SPRY4 (607984) genes in 2 families, respectively. In the first family, a woman with mild cranial dysmorphism had a de novo 1-bp deletion in the SPRY1 gene that was transmitted to her son and daughter, both of whom had sagittal craniosynostosis. In the second family, a de novo SPRY4 nonsense mutation arose in a sporadic patient with sagittal craniosynostosis.
See 601380 for discussion of a possible association of unicoronal synostosis with variation in the ephrin A4 (EFNA4) gene.