Schaaf-Yang Syndrome
A number sign (#) is used with this entry because of evidence that Schaaf-Yang syndrome (SHFYNG) is caused by heterozygous mutation in the MAGEL2 gene (605283) on chromosome 15q11.
DescriptionSHFYNG syndrome is an autosomal dominant multisystem disorder characterized by delayed psychomotor development, impaired intellectual development, hypotonia, and behavioral abnormalities. Additional features include contractures, feeding difficulties, and variable dysmorphic facial features. The severity of the disorder is highly variable: some patients may die in utero with fetal akinesia, whereas others can live with moderate disability. Individuals are affected only if the mutation occurs on the paternal allele, since MAGEL2 is a maternally imprinted gene (summary by Fountain et al., 2017)
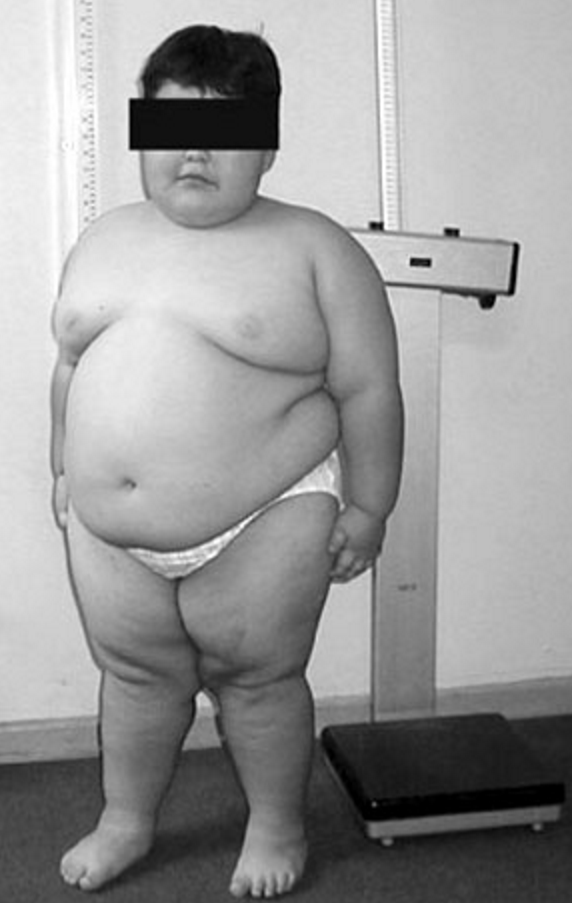
Clinical FeaturesSchaaf et al. (2013) reported 4 unrelated boys with features resembling Prader-Willi syndrome (PWS; 176270). Clinical features that met the major criteria for PWS included neonatal hypotonia with poor suck, feeding problems in infancy, hyperphagia with excessive weight gain before age 6 years, developmental delay, and hypogonadism. Some patients had dysmorphic facial features, such as large mouth, coarse features, almond-shaped palpebral fissures, and bitemporal narrowing. More variable features in these patients that met the PWS minor criteria included short stature, small hands, narrow hands, eye abnormalities, speech defects, skin picking, and sleep apnea. Two patients had temper tantrums or violent outbursts. All had autism spectrum disorder. Two patients had contractures of the proximal and distal interphalangeal joints. Other variable features included constipation, micropenis, and cryptorchidism. One patient had seizures.
Soden et al. (2014) reported 2 sisters, born of unrelated Caucasian parents, with a neurodevelopmental disorder resembling Prader-Willi syndrome. The neonatal course in both patients was complicated by hyperinsulinemic hypoglycemia, poor feeding, respiratory difficulties, and hypotonia. Dysmorphic features included ptosis, exotropia, high palate, smooth philtrum, low muscle mass, short upper arms with decreased elbow extension, and increased central body fat. One patient had seizures. Both girls had global developmental delay and autistic features.
Fountain et al. (2017) reported 18 patients with SHFYNG ascertained on the basis of genetic studies from several different research groups or laboratories. There were 3 families with more than 1 affected individual, including a family with 11 affected who were not all tested molecularly. Combining the phenotypes with previously reported patients yielded a variable yet consistent picture of the clinical course. The patients had hypotonia and delayed psychomotor development ranging from sitting and speaking a few months later than normal to being unable to walk or speak in the late teens. On average, the patients sat independently at 19 months, walked at 39 months, and spoke their first word at 56 months. The level of impaired intellectual development ranged from mild to severe, and 4 patients had a formal diagnosis of autism, although 6 additional patients had symptoms of autism. Several patients had abnormal behavior described as impulsive, compulsive, stubborn, and manipulative, as well as habitual skin picking or automutilation. Two patients had seizures. Dysmorphic features were also common, including abnormal philtrum, ear position, nasal structure, and palpebral fissure length, as well as frontal bossing, prognathism, bushy eyebrows, esotropia, and strabismus. Joint contractures were present in almost all patients, and ranged from only the interphalangeal joints to lethal fetal akinesia with severe arthrogryposis (in patients 5 and 6, who were sibs). Abnormalities of the hands included tapering fingers, clinodactyly, camptodactyly, brachydactyly, and adducted thumbs. Other skeletal features included small hands, small feet, short stature, and scoliosis or kyphosis. Other features included sleep apnea and other sleep abnormalities, feeding difficulties requiring special techniques, and hypogonadism; cryptorchidism and/or micropenis was present in 8 of 11 male patients, and at least one 20-year-old female patient had hypogonadotropic hypogonadism.
Urreizti et al. (2017) reported a 19-year-old Spanish woman (patient 7) who presented at birth with contractures, hypotonia, and respiratory depression. She had premature fusion of the metopic sutures, resulting in trigonocephaly. Severe developmental delay was noted, with walking achieved at age 11 years, severely impaired intellectual development, lack of speech acquisition, and feeding difficulties with poor overall growth. She also had sleep disturbances, temperature instability, excessive salivation and sweating, and sleep apnea with episodic hyperventilation. Dysmorphic features included strabismus, short nose, anteverted nares, macrostomia, thick palatal and alveolar ridges, malpositioned teeth, widely spaced nipples, hypogonadism, asymmetric thorax, and marked lordosis. She was diagnosed clinically with Opitz syndrome C (211750) before genetic analysis confirmed SHFYNG.
Clinical Variability
Mejlachowicz et al. (2015) reported a family in which 3 fetuses, offspring of unrelated parents, died in utero with a severe form of SHFYNG manifest clinically as arthrogryposis multiplex congenita (AMC). Prenatal ultrasound and pathologic examination showed polyhydramnios, decreased fetal movements, clubfoot, joint contractures, and camptodactyly. Additional features included hypertelorism, short palpebral fissures, microretrognathia, and short neck. Morphologic examination of the brain, spinal cord, and neuromuscular junction did not reveal any specific defect. An unrelated child presented with a similar disorder. She had microretrognathia, short neck, clubfeet, camptodactyly, and severe hypotonia with respiratory insufficiency, resulting in death at 2 days of age. Pathologic examination of other organs, including the brain and spinal cord, was normal.
Two of the patients reported by Fountain et al. (2017) were fetal sibs (patients 5 and 6) with SHFYNG manifest as AMC resulting in termination of the pregnancies. The patients had fetal akinesia, overlapping digits, rocker-bottom feet, retromicrognathia, and gnathopalatoschisis. One had multiple pterygia and talipes equinovarus.
InheritanceThe transmission pattern of SHFYNG in the families reported by Fountain et al. (2017) was consistent with autosomal dominant inheritance with the mutation present on the paternal allele, since the MAGEL2 gene is maternally imprinted and expressed only by the paternal allele. Unlike classical autosomal dominant disorders, the SHFYNG phenotype can skip several generations as long as the mutation resides on the maternal (imprinted) chromosome. However, the chance that the offspring of male individuals carrying a deleterious MAGEL2 mutation will be clinically affected is 50%.
Molecular GeneticsIn 4 unrelated boys with a syndrome resembling Prader-Willi syndrome, Schaaf et al. (2013) identified 4 different de novo heterozygous truncating mutations in the MAGEL2 gene (605283.0001-605283.0004). All mutations occurred on the paternal allele. Because the maternal allele is not normally expressed, the findings were consistent with a loss of MAGEL2 function. The mutation in the first patient was found by clinical whole-exome sequencing. Based on these results, a research database of 1,248 whole-exome sequencing cases were reviewed, and the 3 remaining cases were identified.
In 2 sisters with Schaaf-Yang syndrome, Soden et al. (2014) identified a heterozygous truncating mutation in the MAGEL2 gene (605283.0005). The mutation was found by whole-genome sequencing and apparently resulted from gonadal mosaicism; the mutation was missed by initial whole-exome sequencing. The patients were part of a larger cohort of 100 families with neurodevelopmental disorders who underwent whole-exome or whole-genome sequencing.
In 18 patients with SHFYNG, Fountain et al. (2017) identified heterozygous truncating mutations in the MAGEL2 gene (see, e.g., 605283.0005-605383.0006, 605283.0008-605382.0009). The patients were ascertained based on genotype from whole-exome or direct Sanger sequencing through multiple research-based centers or laboratories. All mutations, which were confirmed by Sanger sequencing, resulted in a truncated protein. All patients tested carried the mutation on the paternal allele, consistent with maternal imprinting of the MAGEL2 gene. In 3 families, the mutation segregated with the disorder: unaffected fathers inherited the mutation from an unaffected mother. Fountain et al. (2017) speculated that the mutations could result in a dominant-negative effect. The phenotype was highly variable, ranging from relatively mild contractures to fetal akinesia, AMC, and early death. Nucleotides c.1990-1996 of MAGEL2 include a sequence of 7 cytosines that represent a mutational hotspot: 11 individuals from 7 families had a c.1996dupC mutation (605382.0005), and 2 from the same family had a c.1996delC mutation (605382.0006). Functional studies of the variants and studies of patient cells were not performed.
Genotype/Phenotype CorrelationsIn 3 fetuses, born of unrelated parents, with Schaaf-Yang syndrome manifest as AMC and death in utero, Mejlachowicz et al. (2015) identified a heterozygous truncating mutation in the MAGEL2 gene (c.1996delC; 605283.0006). The mutation, which was found by a combination of linkage analysis and whole-exome sequencing and confirmed by Sanger sequencing, was inherited from the unaffected father who inherited it from his unaffected mother. Direct Sanger sequencing of the MAGEL2 gene in 84 additional cases of AMC and/or decreased fetal motility identified another patient with a de novo heterozygous truncating mutation (c.2118delT; 605283.0007) that occurred on the paternal allele.
Fountain et al. (2017) identified the same heterozygous c.1996delC mutation in 2 fetal sibs (patients 5 and 6) with SHFYNG manifest as AMC resulting in termination of the pregnancies.
Other FeaturesBuiting et al. (2014) reported a 3-year-old boy with a paternally inherited deletion of approximately 3.9 Mb that included MAGEL2, but not the SNRPN (182279)/SNORD116 (605436) locus. Apart from delayed motor skills, the boy was asymptomatic. Buiting et al. (2014) stated that this was the second individual with a MAGEL2 deletion who certainly does not have Prader-Willi syndrome; the first individual was also described by this group (Kanber et al., 2009). The authors concluded that it is important to distinguish between point mutations and whole gene deletions, and that because the effect of the genes in the PWS chromosomal region may be epistatic rather than additive, the role of MAGEL2 in Prader-Willi syndrome remained unclear.
Animal ModelBischof et al. (2007) found that Magel2-null mice showed features similar to those of Prader-Willi syndrome (PWS; 176270) in humans. There was reduced embryonic viability associated with loss of Magel2. Magel2-null mice showed neonatal growth retardation, excessive weight gain after weaning, and increased adiposity with altered metabolism, including increased fasting insulin and elevated cholesterol, in adulthood. Mutant mice also showed abnormalities in the circadian pattern of feeding behavior. The findings implicated loss of the Magel2 gene in hypothalamic dysfunction.
Schaller et al. (2010) reported that a Magel2-deficient mouse strain with 50% neonatal mortality had an altered onset of suckling activity and subsequent impaired feeding, suggesting a role of MAGEL2 in the suckling deficit seen in newborns with PWS. The hypothalamus of Magel2 mutant neonates showed a significant reduction in oxytocin (OT; 167050). Furthermore, injection of a specific oxytocin receptor antagonist in wildtype neonates recapitulated the feeding deficiency seen in Magel2 mutants, and a single injection of oxytocin, 3 to 5 hours after birth, rescued the phenotype of Magel2 mutant pups, allowing all of them to survive. The authors proposed that oxytocin supplementation might constitute a promising treatment for feeding difficulties in PWS neonates and potentially in other newborns with impaired feeding onset.