Fraser Syndrome 2
A number sign (#) is used with this entry because of evidence that Fraser syndrome-2 (FRASRS2) is caused by homozygous mutation in the FREM2 gene (608945) on chromosome 13q13.
Mutation in the FREM2 gene can also cause isolated unilateral or bilateral cryptophthalmos (CRYPTOP; 123570).
DescriptionFraser syndrome is an autosomal recessive malformation disorder characterized by cryptophthalmos, syndactyly, and abnormalities of the respiratory and urogenital tract (summary by van Haelst et al., 2008).
For a general phenotypic description and a discussion of genetic heterogeneity of Fraser syndrome, see 219000.
Clinical FeaturesJadeja et al. (2005) reported 2 unrelated consanguineous families segregating Fraser syndrome. The proband in the first family, who died 1 hour after birth, had cryptophthalmos, nasal dysplasia with hypoplastic alae nasi, multiple skin syndactyly in all extremities, and short thorax with abdominal distention. Autopsy showed that the right kidney and ureter were absent, the left kidney was small and dysplastic and the ureter was directly connected to the vagina, the bladder was absent, and the vagina was dilated with external genitalia of male appearance. The karyotype was 46,XX. The proband in the second family had complete cryptophthalmos of the right eye and a malformed left globe, a small mouth, an abnormally low hair line, and cutaneous syndactyly of both hands and one foot. The external genitalia were ambiguous.
Shafeghati et al. (2008) reported a female fetus, the offspring of consanguineous Iranian parents, with a normal karyotype and cryptophthalmos, ambiguous external genitalia, syndactyly, bilobed lungs, bilateral renal agenesis, hypoplastic bladder, and agenesis of internal genitalia with streak ovaries. An earlier pregnancy in the family had resulted in the intrauterine death at 30 weeks of gestation of a male fetus with a normal karyotype in whom the diagnosis of Fraser syndrome was suggested by the presence of cryptophthalmos, syndactyly, ambiguous genitalia, imperforate anus, bilateral renal agenesis, pulmonary hypoplasia, and hydrocephalus. The authors noted that the findings in the sibs were consistent with classic Fraser syndrome.
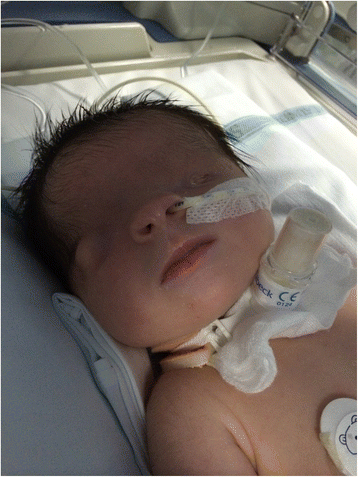
Yu et al. (2018) studied an 11-month old Chinese girl (patient 2) who had left renal agenesis noted on prenatal ultrasound. At birth, she had right-sided complete cryptophthalmia, and renal ultrasound confirmed left renal agenesis. She had no syndactyly, mental retardation, omphalocele, or other congenital defects of ears, nose, larynx, or anus.
MappingIn 3 families segregating Fraser syndrome unlinked to the FRAS1 gene (607830), Jadeja et al. (2005) found linkage of the disorder to chromosome 13q13.3.
Molecular GeneticsIn 2 unrelated consanguineous families with Fraser syndrome linked to chromosome 13q13.3, Jadeja et al. (2005) found a homozygous missense mutation in the FREM2 gene (E1972K; 608945.0001). The authors noted that in 1 family, of Spanish Gypsy origin, the parents were heterozygous for the mutation.
In a female fetus with Fraser syndrome, offspring of a consanguineous Iranian couple, Shafeghati et al. (2008) identified homozygosity for a splice site mutation in the FREM2 gene (608945.0002). The parents were heterozygous for the mutation.
In an 11-month-old Chinese girl (patient 2) with unilateral cryptophthalmos and left renal agenesis, Yu et al. (2018) sequenced the FRAS1, FREM2, FREM1 (608944), and GRIP1 (604597) genes, and identified compound heterozygosity for mutations in the FREM2 gene: a missense mutation (R2167W; 608945.0004) and a 1-bp deletion (608945.0005). The mutations segregated with disease and were not found in an in-house database of 8,000 Chinese control exomes.
Animal ModelTo investigate the cause of Fraser syndrome not linked to FRAS1, Jadeja et al. (2005) carried out linkage analysis in the mouse 'myelencephalic blebs' (my) strain, which shows a phenotype similar to that of Fras1-mutant mice. The authors mapped the my phenotype to the Frem2 gene and showed that a Frem2 gene trap mutation was allelic to my.
Kiyozumi et al. (2006) found that my/my mice had reduced localization of Frem2, as well as Fras1 and Frem1 (608944), to epidermal basement membranes. Frem1-knockout mice had reduced expression of Fras1 and Frem2 at the basement membrane. When coexpressed and secreted by transfected cells, these proteins formed a ternary complex, raising the possibility that their reciprocal stabilization at the basement membrane was due to complex formation. Kiyozumi et al. (2006) suggested that coordinated assembly of the 3 Fraser syndrome-associated proteins at the basement membrane is instrumental in epidermal-dermal interactions during morphogenetic processes.