Hpv-Positive Oropharyngeal Cancer

Human papillomavirus-positive oropharyngeal cancer (HPV-positive OPC or HPV+OPC), is a cancer (squamous cell carcinoma) of the throat caused by the human papillomavirus type 16 virus (HPV16). In the past, cancer of the oropharynx (throat) was associated with the use of alcohol or tobacco or both, but the majority of cases are now associated with the HPV virus, acquired by having oral contact with the genitals (oral-genital sex) of a person who has a genital HPV infection. Risk factors include having a large number of sexual partners, a history of oral-genital sex or anal–oral sex, having a female partner with a history of either an abnormal Pap smear or cervical dysplasia, having chronic periodontitis, and, among men, younger age at first intercourse and a history of genital warts. HPV-positive OPC is considered a separate disease from HPV-negative oropharyngeal cancer (also called HPV negative-OPC and HPV-OPC).
HPV-positive OPC presents in one of four ways: as an asymptomatic abnormality in the mouth found by the patient or a health professional such as a dentist; with local symptoms such as pain or infection at the site of the tumor; with difficulties of speech, swallowing, and/or breathing; or as a swelling in the neck if the cancer has spread to local lymph nodes. Detection of a tumour suppressor protein, known as p16, is commonly used to diagnose an HPV associated OPC. The extent of disease is described in the standard cancer staging system, using the AJCC TNM system, based on the T stage (size and extent of tumor), N stage (extent of involvement of regional lymph nodes) and M stage (whether there is spread of the disease outside the region or not), and combined into an overall stage from I–IV. In 2016, a separate staging system was developed for HPV+OPC, distinct from HPV-OPC.
Whereas most head and neck cancers have been declining as reduced smoking rates have declined, HPV-positive OPC has been increasing. Compared to HPV-OPC patients, HPV-positive patients tend to be younger, have a higher socioeconomic status and are less likely to smoke. In addition, they tend to have smaller tumours, but are more likely to have involvement of the cervical lymph nodes. In the United States and other countries, the number of cases of oropharyngeal cancer has been increasing steadily, with the incidence of HPV-positive OPC increasing faster than the decline in HPV-negative OPC. The increase is seen particularly in young men in developed countries, and HPV-positive OPC now accounts for the majority of all OPC cases. Efforts are being made to reduce the incidence of HPV-positive OPC by introducing vaccination that includes HPV types 16 and 18, found in 95% of these cancers, prior to exposure to the virus. Early data suggest a reduction in infection rates.
In the past, the treatment of OPC was radical surgery, with an approach through the neck and splitting of the jaw bone, which resulted in morbidity and poor survival rates. Later, radiotherapy with or without the addition of chemotherapy, provided a less disfiguring alternative, but with comparable poor outcomes. Now, newer minimally invasive surgical techniques through the mouth have improved outcomes; in high risk cases, this surgery is often followed by radiation and/or chemotherapy. In the absence of high quality evidence regarding which treatment provides the best outcomes, management decisions are often based on one or more of the following: technical factors, likely functional loss, and patient preference. The presence of HPV in the tumour is associated with a better response to treatment and a better outcome, independent of the treatment methods used, and a nearly 60% reduced risk of dying from the cancer. Most recurrence occurs locally and within the first year after treatment. The use of tobacco decreases the chances of survival.
Signs and symptoms
HPV+OPC presents in one of four ways: as an asymptomatic abnormality in the mouth found by the patient or a health professional such as a dentist; with local symptoms such as pain or infection at the site of the tumor; with difficulties of speech, swallowing, and/or breathing; or as a swelling in the neck (if the cancer has spread to lymph nodes). These may be accompanied by more general symptoms such as loss of appetite, weight loss, and weakness.
Cause

Most mucosal squamous cell head and neck cancers, including oropharyngeal cancer (OPC), have historically been attributed to tobacco and alcohol use. However this pattern has changed considerably since the 1980s. It was realised that some cancers occur in the absence of these risk factors and an association between human papilloma virus (HPV) and various squamous cell cancers, including OPC, was first described in 1983. Since then both molecular and epidemiological evidence has been accumulating, with the International Agency for Research on Cancer (IARC) stating that high-risk HPV types 16 and 18 are carcinogenic in humans, in 1995, and In 2007 that HPV was a cause for oral cancers. Human papillomavirus (HPV)-positive cancer (HPV+OPC) incidence has been increasing while HPV-negative (HPV-OPC) cancer incidence is declining, a trend that is estimated to increase further in coming years. Since there are marked differences in clinical presentation and treatment relative to HPV status, HPV+OPC is now viewed as a distinct biologic and clinical condition.
Human HPV has long been implicated in the pathogenesis of several anogenital cancers including those of the anus, vulva, vagina, cervix, and penis. In 2007 it was also implicated by both molecular and epidemiological evidence in cancers arising outside of the anogenital tract, namely oral cancers. HPV infection is common among healthy individuals, and is acquired through oral sex. Although less data is available, prevalence of HPV infection is at least as common among men as among women, with 2004 estimates of about 27% among US women aged 14–59.
HPV oral infection precedes the development of HPV+OPC. Slight injuries in the mucous membrane serve as an entry gate for HPV, which thus works into the basal layer of the epithelium. People testing positive for HPV type 16 virus (HPV16) oral infection have a 14 times increased risk of developing HPV+OPC. Immunosuppression seems to be an increased risk factor for HPV+OPC. Individuals with TGF-β1 genetic variations, specially T869C, are more likely to have HPV16+OPC. TGF-β1 plays an important role in controlling the immune system. In 1993 it was noted that patients with human papillomavirus (HPV)-associated anogenital cancers had a 4-fold increased risk of tonsillar squamous-cell carcinoma. Although evidence suggests that HPV16 is the main cause of OPC in humans not exposed to smoking and alcohol, the degree to which tobacco and/or alcohol use may contribute to increase the risk of HPV+OPC has not always been clear but it appears that both smoking and HPV infection are independent and additive risk factors for developing OPC. The connection between HPV-infection and oropharyngeal cancer is stronger in regions of lymphoepithelial tissue (base of tongue and palatine tonsils) than in regions of stratified squamous epithelium (soft palate and uvula). Human herpesvirus-8 infection can potentiate the effects of HPV-16.
Risk factors
Risk factors include a high number of sexual partners (25% increase >= 6 partners), a history of oral-genital sex (125% >= 4 partners), or anal–oral sex, a female partner with a history of either an abnormal Pap smear or cervical dysplasia, chronic periodontitis, and, among men, decreasing age at first intercourse and history of genital warts.
Pathology

Cancers of the oropharynx primarily arise in lingual and palatine tonsil lymphoid tissue that is lined by respiratory squamous mucosal epithelium, which may be invaginated within the lymphoid tissue. Therefore, the tumour first arises in hidden crypts. OPC is graded on the basis of the degree of squamous and keratin differentiation into well, moderate or poorly (high) differentiated grades. Other pathological features include the presence of finger-like invasion, perineural invasion, depth of invasion and distance of the tumour from resection margins. Phenotypic variants include basaloid squamous carcinoma, a high grade form (see Chung Fig. 35-3(C) and illustration here). They are most commonly non-keratinising. HPV+OPC also differs from HPV-OPC in being focal rather than multifocal and not being associated with pre-malignant dysplasia. HPV+OPC patients are therefore at less risk of developing other malignancies in the head and neck region, unlike other head and neck primary tumours that may have associated second neoplasms, that may occur at the same time (synchronous) or a distant time (metachronous), both within the head and neck region or more distantly. This suggests that the oncogenic alterations produced by the virus are spatially limited rather than related to a field defect.
Anatomy
The oropharynx, at the back of the mouth, forms a circle and includes the base of the tongue (posterior third) below, the tonsils on each side, and the soft palate above, together with the walls of the pharynx, including the anterior epiglottis, epiglottic valleculae and branchial cleft at its base. The oropharynx is one of three divisions of the interior of the pharynx based on their relation to adjacent structures (nasal pharynx (nasopharynx), oral pharynx (oropharynx) and laryngeal pharynx (laryngopharynx - also referred to as the hypopharynx), from top to bottom). The pharynx is a semicircular fibromuscular tube joining the nasal cavities above to the larynx (voice box) and oesophagus (gullet), below, where the larynx is situated in front of the oesophagus.
The oropharynx lies between the mouth (oral cavity) to the front, and the laryngopharynx below, which separates it from the larynx. The upper limit of the oropharynx is marked by the soft palate, and its lower limit by the epiglottis and root of the tongue. The oropharynx communicates with the mouth, in front through what is known as the oropharyngeal isthmus, or isthmus of the fauces. The isthmus (i.e. connection) is formed above by the soft palate, below by the posterior third of the tongue, and at the sides by the palatoglossal arches. The posterior third of the tongue, or tongue base contains numerous follicles of lymphatic tissue that form the lingual tonsils. Adjacent to the tongue base, the lingual surface of the epiglottis, which curves forward, is attached to the tongue by median and lateral glossoepiglottic folds. The folds form small troughs known as the epiglottic valleculae. The lateral walls are marked by two vertical pillars on each side, the pillars of the fauces, or palatoglossal arches. More properly they are separately named the palatoglossal arch anteriorly and the palatopharyngeal arch posteriorly. The anterior arch is named from the palatoglossal muscle within, running from the soft palate to the tongue (glossus), while the posterior arch similarly contains the palatopharyngeal muscle running from the soft palate to the lateral pharynx. Between the arches lies a triangular space, the tonsillar fossa in which lies the palatine tonsil, another lymphoid organ.
The external pharyngeal walls consisting of the four constrictor muscles form part of the mechanism of swallowing. The microscopic anatomy is composed of four layers, being from the lumen outwards, the mucosa, submucosa, muscles and the fibrosa, or fibrous layer. The mucosa consists of stratified squamous epithelium, that is generally non-keratinised, except when exposed to chronic irritants such as tobacco smoke. The submucosa contains aggregates of lymphoid tissue.
Patterns of spread
Cancers arising in the tonsillar fossa spread to the cervical lymph nodes, primarily the subdigastric (upper jugular) lymph nodes (level II), with secondary involvement of the mid (level III) and low (level IV) jugular nodes and sometimes the posterior cervical nodes (level V). Base of tongue cancers spread to the subdigastric and mid jugular nodes, and occasionally posterior cervical nodes but being closer to the midline are more likely to have bilateral nodal disease. Tonsillar cancers rarely spread to the contralateral side unless involving the midline.
Mechanism
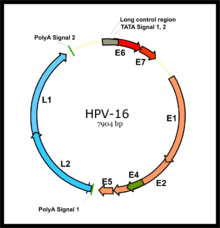
Virology
HPV associated cancers are caused by high-risk strains of HPV, mainly HPV-16 and HPV-18. HPV is a small non-enveloped DNA virus of the papillomavirus family. Its genome encodes the early (E) oncoproteins E5, E6 and E7 and the late (L) capsid proteins L1 and L2. The virus gains access to the mucosa through microlesions, where it infects the basal layer of cells, which are still able to proliferate. While the virus does not replicate in these cells, expression of its early genes stimulates proliferation and lateral expansion of the basal cells. As this moves the virus particles into the overlying suprabasal layers, late viral gene expression occurs, enabling replication of the circular viral genome (see figure) and structural proteins. As these are pushed into the most superficial mucosal layers, complete viral particles are assembled and released.
Oncogenesis
An increased risk of HPV+OPC is observed more than 15 years after HPV exposure, pointing to a slow development of the disease, similar to that seen in cervical cancer. Relative to HPV-OPC, the oncogenic molecular progression of HPV+OPC is poorly understood. The two main viral oncoproteins of the high risk HPV types are E6 and E7. These are consistently expressed in malignant cell lines, and if their expression is inhibited the malignant phenotype of the cancer cells is blocked. Either of these oncoproteins can immortalise cell lines, but are more efficient when both are expressed, since their separate molecular roles are synergistic. The E6 and E7 oncogenes become integrated into host-cell DNA, and the oncoproteins they express interfere with a variety of predominantly antiproliferative cellular regulatory mechanisms. They bind to and inactivate the best known of these mechanisms, the tumor suppressor proteins p53 and retinoblastoma protein pRB (pRb) leading to genomic instability and then cell cycle deregulation (see Chung et al., 2016 Fig. 35.2). Further, yet to be elicited, mechanisms are required for the final steps of malignant transformation of HPV infected cells.
HPV- and HPV+OPC are distinguishable at the molecular level. The naturally occurring (wild type) p53 is widely involved in cellular processes, including autophagy, response to DNA damage, cell cycle regulation and senescence, apoptosis and the generation of adenosine triphosphate (ATP) through oxidative phosphorylation. The gene encoding p53 is inactivated by E6 at the protein level and is found as the wild type in HPV+OPC but mutated in HPV-OPC. In HPV+OPC p53 protein undergoes accelerated degradation by E6, drastically reducing its levels, while in HPV-OPC it undergoes genetic mutation, which may result in synthesis of an abnormal p53 protein, that may not only be inactive as a tumour suppressor, but can also bind and inactivate any non-mutated wild type p53, with an increase in oncogenic activity. Although p53 mutations occur in HPV+OPC, they are far less common than in HPV-OPC (26% vs 48%), and do not appear to affect clinical outcome.
The pRb protein is inactivated by E7 in HPV+OPC, but in HPV-OPC it is the p16 tumour suppressor part of the pRb tumour suppressor network that is inactivated. Also the pRb pathway is inactivated by E7 instead of Cyclin D1 amplification. CDKN2A is a tumour suppressor gene that encodes a tumor suppressor protein, p16 (cyclin-dependent kinase inhibitor 2A) and inhibits the kinase activity of the cyclin-dependent kinases CDK4 and CDK6, which in turn induce cell cycle arrest. p16 expression is cell cycle dependent and is expressed focally in only about 5–10% of normal squamous epithelium. Like most HPV+ cancers, HPV+OPC express p16 but the latter does not function as a tumour-suppressor, because the mechanism by which this is achieved, pRb, has been inactivated by E7. p16 is upregulated (over-expressed) due to E7-related loss of pRB with reduced negative feedback, whereas it is downregulated in up to 90% of HPV-OPC. This diffuse over-expression in the tumour cells provides a diagnostic marker for HPV involvement. Although HPV E6 and E7 reduce tumour suppressor activity, they do so less than genetic and epigenetic processes do in HPV-OPC.
The tonsillar epithelia (palatine and lingual) share similar nonkeratinization characteristics with the cervix, where HPV infection plays the major role in cases of cervical cancer. Also E6 and E7 may make HPV+OPC more immunogenic than HPV-OPC, since anti-E6 and E7 antibodies may be detected in these patients. This in turn could restrict the malignant behaviour of HPV+OPC and the presence of antibodies has been associated with a better prognosis, while treatment may enhance the immunogenicity of the tumour, and hence improve response, although to what extent is not clear. Outcomes are also associated with improved adaptive immunity.
Diagnosis


Biopsy
Initial diagnosis requires visualisation of the tumour either through the mouth or endoscopically through the nose using a rhinoscope, illustrated to the right, followed by biopsy.
Differentiating HPV+OPC from HPV-OPC
HPV+OPC is usually diagnosed at a more advanced stage than HPV-OPC, with 75–90% having involvement of regional lymph nodes. Furthermore, nonkeratinizing squamous cell carcinoma is strongly associated with HPV-OPC.
Genetic signatures of HPV+ and HPV- OPC are different. HPV+OPC is associated with expression level of the E6/E7 mRNAs and of p16. HPV16 E6/E7-positive cases are histopathologically characterized by their verrucous or papillary (nipple like) structure and koilocytosis of the adjacent mucosa. Approximately 15% of HNSCCs are caused by HPV16 infection and the subsequent constitutive expression of E6 and E7, and some HPV-initiated tumors may lose their original characteristics during tumor progression. High-risk HPV types may be associated with oral carcinoma, by cell-cycle control dysregulation, contributing to oral carcinogenesis and the overexpression of mdm2, p27 and cathepsin B.
HPV+OPC is not merely characterized by the presence of HPV-16: only the expression of viral oncogenes within the tumor cells plus the serum presence of E6 or E7 antibodies is unambiguously conclusive for HPV+OPC.
There is not a standard HPV testing method in head and neck cancers, both in situ hybridization (ISH) and polymerase chain reaction (PCR) are commonly used. Both methods have comparable performance for HPV detection, however it is important to use appropriate sensitivity controls. Immunohistochemistry (IHC) staining of the tissue for p16 is frequently used as a cost-effective surrogate for HPV in OPC, compared to ISH or PCR but there is a small incidence of HPV-negative p16-positive disease accounting for about 5% of HPV-OPC.
Staging
Staging is generally by the UICC/AJCC TNM (Tumour, Nodes, Metastases) system. Staging is based on clinical examination, diagnostic imaging, and pathology. On imaging, involved lymph nodes may appear cystic, a characteristic of HPV+OPC.
HPV+OPC has been treated similarly to stage-matched and site-matched HPV unrelated OPC, but its unique features, which contrast smoking-related HPV-OPC head and neck cancers, for which patients' demographics, comorbidities, risk factors, and carcinogenesis differ markedly, suggest that a distinct staging system be developed to more appropriately represent the severity of the disease and its prognosis. Standard AJCC TNM staging, such as the seventh edition (2009) while predictive for HPV-OPC has no prognostic value in HPV+OPC. The 8th edition of the AJCC TNM Staging Manual (2016) incorporates this specific staging for HPV+OPC. As of 2018, treatment guidelines are evolving to account for the different outcomes observed in HPV+OPC. Consequently, less intensive (de-intensification) use of radiotherapy or chemotherapy, as well as specific therapy, is under investigation, enrolling HPV+OPC in clinical trials to preserve disease control and minimise morbidity in selected groups based on modified TNM staging and smoking status.
HPV+ cancer of the oropharynx are staged as (AJCC 8th ed. 2016): Tumour stage
- T0 no primary identified
- T1 2 cm or less in greatest dimension
- T2 2–4 cm
- T3 >4 cm, or extension to lingual surface of epiglottis
- T4 moderately advanced local disease, invading larynx, extrinsic muscle of tongue, medial pterygoid, hard palate, or mandible or beyond
Nodal stage
- Nx regional lymph nodes cannot be assessed
- N0 no regional lymph nodes involved
- N1 one or more ipsilateral nodes involved, less than 6 cm
- N2 contralateral or bilateral lymph nodes, less than 6 cm
- N3 lymph node(s) larger than 6 cm
Clinical stage
- Stage I: T0N1, T1–2N0–1
- Stage II: T0N2, T1–3N2, T3N0–2
- Stage III: T0–3N3, T4N0-3
- Stage IV: any metastases (M1)
However, the published literature and ongoing clinical trials use the older seventh edition that does not distinguish between HPV+OPC and HPV-OPC - see Oropharyngeal Cancer - Stages. The T stages are essentially similar between AJCC 7 and AJCC 8. with two exceptions. Tis (carcinoma in situ) has been eliminated and the division of T4 into substages (e.g. T4a) has been removed. The major changes are in the N stages, and hence the overall clinical stage. N0 remains the same, but as with the T stage, substages such as N2a have been eliminated. Extracapsular extension (ECE), also referred to as extranodal extension (ENE), which is invasion by the tumour beyond the capsule of the lymph node has been eliminated as a staging criterion.
This results in a HPV+OPC tumour being given a lower stage than if it were HPV-OPC. For instance, a 5 cm tumour with one ipsilateral node involved that is 5 cm in size but has ECE would be considered T3N3bM0 Stage IVB if HPV- but T3N1M0 Stage II if HPV+.
Prevention

Avoiding exposure
Prevention of HPV+OPC involves avoiding or reducing exposure to risk factors where possible.
Vaccination
About 90% of HPV+OPC carry HPV 16, and another 5% type 18. These two types are both targets of available vaccines. HPV vaccines given prior to exposure can prevent persistent genital infection and the consequent precancerous state. Therefore, they have a theoretical potential to prevent oral HPV infection. A 2010 review study has found that HPV16 oral infection was rare (1.3%) among the 3,977 healthy subjects analyzed.
Treatment
The goals of treatment are to optimise survival and locoregional disease control, and prevent spread to distant areas of the body (metastasis), while minimising short and long term morbidity. There is no high quality Level I evidence from prospective clinical trials in HPV+OPC, therefore treatment guidelines must rely on data from treatment of OPC in general and from some retrospective unplanned subsetting of those studies, together with data for head and neck cancer in general. Treatment for OPC has traditionally relied on radiotherapy, chemotherapy and/or other systemic treatments, and surgical resection. Depending on stage and other factors treatment may include a combination of modalities. The mainstay has been radiotherapy in most cases. a pooled analysis of published studies suggested comparable disease control between radiation and surgery, but higher complication rates for surgery +/- radiation. Ideally a single modality approach is preferred, since triple modality is associated with much more toxicity, and a multidisciplinary team in a large centre with high patient volumes is recommended.
Differences in response to treatment between HPV-OPC and HPV+OPC may include differences in the extent and manner in which cellular growth-regulatory pathways are altered in the two forms of OPC. For instance in HPV+OPC the HPV E6 and E7 oncogenes merely render the p53 and pRb pathways dormant, leaving open the possibility of reactivation of these pathways by down-regulating (reducing) expression of the oncogenes. This is in contrast to the mutant form of p53 found in HPV-OPC that is associated with treatment resistance. Furthermore, it is suggested that the effects of E6 and E7 on these pathways renders the tumour more radiosensitive, possibly by interference with mechanisms such as DNA repair, repopulation signalling, and cell-cycle redistribution. The microenvironment is also important, with radiation increasing host immune response to viral antigens expressed on the tumour. Also, there is an association between an increase in tumour-infiltrating lymphocytes and in circulating white blood cells in HPV+OPC patients and better prognosis. This implies a role for an adaptive immune system in suppressing tumour progression.
Surgery
Historically, surgery provided the single approach to head and neck cancer. Surgical management of OPC carried significant morbidity with a transcervical (through the neck) approach, often involving mandibulotomy, in which the jawbone (mandible) is split. This is referred to as an open surgical technique. Consequently, surgical approaches declined in favour of radiation. In the United States, the use of surgery declined from 41% of cases in 1998 to 30% by 2009, the year that the Food and Drug Administration approved the use of the newer techniques.
These improvements in surgical techniques have allowed many tumours to be resected (removed) by transoral (through the mouth) surgical approaches (TOS), using transoral endoscopic head and neck surgery (HNS). Consequently, surgery became used more, increasing to 35% of cases by 2012. This approach has proven safety, efficacy and tolerability, and includes two main minimally invasive techniques, transoral robotic surgery (TORS) and transoral laser microsurgery (TLM). No direct comparisons of these two techniques have been conducted, and clinical trials in head and neck cancer such as ECOG 3311 allow either. They are associated with substantial postoperative morbidity, depending on extent of resection but compared to older techniques have shorter hospital stay, faster recovery, less pain, and less need for gastrostomy or tracheostomy, and less long term effects, which are minimal in the absence of postoperative radiation (RT), or chemoradiation (CRT). TORS has the practical advantage that angled telescopes and rotating robotic surgical arms provide better line of sight. Outcomes of minimally invasive procedures also compare favourably with more invasive ones. In early stage disease, including involvement of neck nodes, TORS produces a 2-year survival of 80–90%. TLM similarly, is reported to have a five-year survival of 78% and local control rates of 85–97%. In addition to early disease, minimally invasive surgery has been used in advanced cases, with up to 90% local control and disease specific survival. Postoperative swallowing was excellent in 87%, but long term dysphagia was associated with larger (T4) cancers, especially if involving the base of the tongue.
The details of the surgical approach depend on the location and size of the primary tumour and its N stage. Neck dissection to examine the draining lymph nodes may be carried out simultaneously or as a second staging procedure. For tumours of the tonsil and lateral pharyngeal wall, and clinically node negative (N0) disease, dissection of the neck typically involves levels 2–4 (see diagram in Dubner 2017) ipsilaterally. Where nodes are involved clinically, dissection will depend on the location and size of the node or nodes. In the case of tongue base primaries, close to the midline, bilateral dissection is recommended.
Pathological staging
An advantage of a primary surgical approach is the amount of pathological information made available, including grade, margin status, and degree of involvement of lymph nodes. This may change the staging, as up to 40% of patients may have a different postoperative pathological stage compared to their preoperative clinical stage. In one study, 24% had their stage reduced (downstaged), which may impact subsequent decision making, including reduction in intensity and morbidity. In the United Kingdom, the Royal College of Pathologists (1998) has standardised the reporting of surgical margins, with two categories, "mucosal" and "deep", and for each created groups based on the microscopic distance from invasive cancer to the margin, as follows: more than 5 mm (clear), 1–5 mm (close) and less than 1 mm (involved).
Adjuvant postoperative therapy
Data on the use of postoperative radiation therapy (PORT) is largely confined to historical or retrospective studies rather than high quality randomized clinical trials and are based on the overall population of patients with head and neck cancer, rather than specific studies of HPV+OPC, which would have formed a very small proportion of the population studied. Despite surgical excision, in the more advanced cases local and regional recurrence of the cancer, together with spread outside of the head and neck region (metastases) are frequent. The risk of subsequent recurrent disease has been considered highest in those tumours where the pathology shows tumour at the margins of the resection (positive margins), multiple involved regional lymph nodes and extension of the tumour outside of the capsule of the lymph node (extracapsular extension), based on historical experience with head and neck cancer. PORT was introduced in the 1950s in an attempt to reduce treatment failure from surgery alone. Although never tested in a controlled setting, PORT has been widely adopted for this purpose. In an analysis of surgical treatment failure at Memorial Sloan-Kettering Cancer Center, patients treated with surgery alone between 1960–1970 had failure rates of 39 and 73% for those with negative and positive surgical margins respectively. These were compared to those who received PORT (with or without chemotherapy) from 1975–1980. The latter group had lower failure rates of 2% and 11% respectively. In addition, one randomised study from the 1970s (RTOG 73-03) compared preoperative radiation to PORT, and found lower failure rates with the latter.
The addition of another modality of treatment is referred to as adjuvant (literally helping) therapy, compared to its use as the initial (primary) therapy, also referred to as radical therapy. Consequently, many of these patients have been treated with adjuvant radiation, with or without chemotherapy. In the above series of reports of minimally invasive surgery, many (30–80%) patients received adjuvant radiation. However, functional outcomes were worse if radiation was added to surgery and worst if both radiation and chemotherapy were used. Radiation dosage has largely followed that derived for all head and neck cancers, in this setting, based on risk. Historically only one randomised clinical trial has addressed optimal dosage, allocated patients to two dosage levels, stratified by risk, but showed no difference in cancer control between the low and high doses (63 and 68.4 Gy), but a higher incidence of complications at the higher doses. Consequently, the lower dose of 57.6 Gy was recommended. Because the authors used a fractionation scheme of 1.8 Gy per treatment, this dosage was not widely adopted, practitioners preferring a larger fraction of 2 Gy to produce a shorter treatment time, and a slightly higher dose of 60 Gy in 2 Gy fractions (30 daily treatments). Yet 57.6 Gy in 1.8 Gy fractions is equivalent (iso-effective dose) to only 56 Gy in 2 Gy fractions. 60 Gy corresponds to the 63 Gy used as the low dose in the high risk group. 60 Gy was also the dose used in RTOG 73-03. Subsequently, there was a tendency to intensify treatment in head and neck cancer, and a number of centres adopted a dose of 66 Gy, at least for those patients with adverse tumour features. The effectiveness of PORT in HPV+OPC receives some support from a cohort study (Level 2b), although the number of patients was low, and the number of events (recurrent disease or death) only 7%. Another retrospective population-level study (Level 4) of the SEER database (1998–2011) concluded that there was an overall survival but not disease-specific survival effect of radiation in 410 patients with a single lymph node involved, but used only univariate statistical analysis and contained no information on HPV status. A subsequent much larger study on a similar population in the National Cancer Database (2004–2013) of over 9,000 patients found a survival advantage but this was only in HPV-OPC, not in 410 HPV+OPC patients, and a subsequent study of 2,500 low and intermediate risk HPV+OPC patients showed similar overall survival whether PORT was given or not.
Deintensification
While less studies have been completed examining deintensification (de-escalation) in this setting, than in primary radical radiation for this cancer (see below), it is an area of active investigation. In one single institution study, a decision was made to reduce the radiation dose in high risk patients with HPV+OPC from 66 to 60 Gy, corresponding to the actual evidence, and follow up has shown no decrease in cancer control. Current trials, both in North America and Europe (such as ECOG 3311 and PATHOS) use 50 Gy as the comparison arm. The comparator of 50 Gy was chosen on the grounds of (i) the exquisite sensitivity of HPV+OPC to radiation, both in vitro and in vivo; ECOG 1308 showing excellent disease control at 54 Gy; and data suggesting that 50 Gy in 1.43 Gy (iso-effective dose 43 Gy in 2.0 Gy) was sufficient to electively treat the neck. Other studies, such as MC1273 and DART-HPV have evaluated doses as low as 30–36 Gy. Lowering the radiation dose to 54 Gy was identified as one of the important Clinical Cancer Advances of 2018 by the American Society of Clinical Oncology, under the general theme of "Less Is More: Preserving Quality of Life With Less Treatment". Chemotherapy has been used concurrently with radiation in this setting, as in primary treatment with radical radiation, particularly where pathological features indicated a higher risk of cancer recurrence. A number of studies have suggested that this does not improve local control, although adding toxicity.
Radiotherapy



Concerns over the morbidity associated with traditional open surgical en-bloc resection, led to exploring alternative approaches using radiation. Intensity modulated radiation therapy (IMRT) can provide good control of primary tumours while preserving excellent control rates, with reduced toxicity to salivary and pharyngeal structures relative to earlier technology. HPV+OPC has shown increased sensitivity to radiation with more rapid regression, compared to HPV-OPC. Generally, radiation can safely be delivered to the involved side alone (ipsilateral), due to the low rate of recurrent cancer on the opposite side (contralateral), and significantly less toxicity compared to bilateral treatment. IMRT has a two-year disease free survival between 82 and 90%, and a two-year disease specific survival up to 97% for stage I and II.
Reported toxicities include dry mouth (xerostomia) from salivary gland damage, 18% (grade 2); difficulty swallowing (dysphagia) from damage to the constrictor muscles, larynx and oesophageal sphincter, 15% (grade 2); subclinical aspiration up to 50% (reported incidence of aspiration pneumonia approximately 14%); hypothyroidism 28–38% at three years (may be up to 55% depending on amount of the thyroid gland exposed to over 45 Gy radiation; esophageal stenosis 5%; osteonecrosis of the mandible 2.5%; and need for a gastrostomy tube to be placed at some point during or up to one year after treatment 4% (up to 16% with longer follow up). Concerns have been expressed regarding excessive short and long term toxicity, especially dysphagia and xerostomia, and hence whether standard doses expose patients with better prognoses are being exposed to overtreatment and unnecessary side effects.
Dosimetry
The probability of xerostomia at one year increases by 5% for every 1Gy increase in dose to the parotid gland. Doses above 25–30 Gy are associated with moderate to severe xerostomia. Similar considerations apply to the submandibular gland, but xerostomia is less common if only one parotid gland is included in the radiated field and the contralateral submandibular gland is spared (less than 39 Gy) In the same manner, radiation dose to the pharyngeal constrictor muscles, larynx, and cricopharyngeal inlet determine the risk of dysphagia (and hence dependence on gastrostomy tube feeds). The threshold for this toxicity is volume-dependent at 55–60 Gy, with moderate to severe impairment of swallowing, including aspiration, stricture and feeding tube dependence above a mean dose of 47 Gy, with a recommended dose to the inferior constrictor of less than 41 Gy. Dose-toxicity relationships for the superior and middle constrictors are steep, with a 20% increase in the probability of dysphagia for each 10 Gy. For late dysphagia, threshold mean total constrictor doses, to limit rates of greater than or equal to grade 2 and 3 below 5% were 58 and 61 Gy respectively. For grade 2 dysphagia, the rate increased by 3.4% per Gy. Doses above 30 Gy to the thyroid are associated with moderate to severe hypothyroidism. Subjective, patient-reported outcomes of quality of life also correlate with radiation dose received.
Altered fractionation schemes, such as RTOG 9003 and RTOG 0129 have not conferred additional benefit. Radiation dose recommendations were largely determined empirically in clinical studies with few HPV+OPC patients, and have remained unchanged for half a century, making it difficult to determine the optimum dose for this subgroup. A common approach uses 70 Gy bilaterally and anteriorly, such as RTOG 9003 (1991–1997) and RTOG 0129 (2002–2005). For lateralized tonsil cancer unilateral neck radiation is usually prescribed, but for tongue base primaries bilateral neck radiation is more common, but unilateral radiation may be used where tongue base lesions are lateralised.
Deintensification
Concerns have been expressed regarding excessive short and long term toxicity, especially dysphagia and xerostomia, and hence whether standard doses expose patients with better prognoses to overtreatment and unnecessary side effects. Current toxicities have been described as "not tolerable", and hence an intense interest in de-escalation.
While comparison with historical controls has limited value compared to randomised clinical trials (phase


