Pfeiffer Syndrome
A number sign (#) is used with this entry because Pfeiffer syndrome can be caused by heterozygous mutations in the FGFR1 gene (136350) on chromosome 8 or in the FGFR2 gene (176943) on chromosome 10. Some families do not map to either of these loci by linkage studies, suggesting additional genetic heterogeneity.
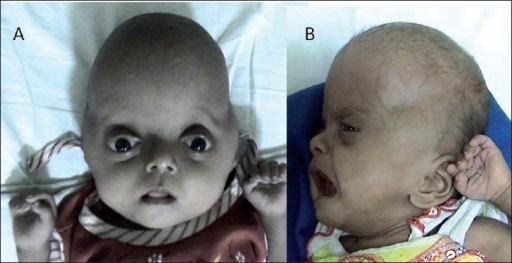
DescriptionPfeiffer syndrome is an autosomal dominant craniosynostosis syndrome with characteristic anomalies of the hands and feet. Three clinical subtypes, which have important diagnostic and prognostic implications, have been identified. Type 1, the classic syndrome, is compatible with life and consists of craniosynostosis, midface deficiency, broad thumbs, broad great toes, brachydactyly, and variable syndactyly. Type 2 consists of cloverleaf skull with Pfeiffer hands and feet, together with ankylosis of the elbows. Type 3 is similar to type 2 but without cloverleaf skull. Ocular proptosis is severe, and the anterior cranial base is markedly short. Various visceral malformations have been found in association with type 3. Early demise is characteristic of types 2 and 3 (Cohen, 1993). Cohen and Barone (1994) further tabulated the findings in the 3 types of Pfeiffer syndrome.
Clinical FeaturesPfeiffer (1964) found 8 persons affected in 3 generations, with 2 instances of male-to-male transmission. The striking feature was broad, short thumbs and big toes. The proximal phalanx of the thumb was either triangular or trapezoid (and occasionally fused with the distal phalanx) so that the thumb pointed outward (i.e., away from the other digits). Martsolf et al. (1971) described the case of an affected boy whose mother and maternal half brother were said to be affected also. Another pedigree consistent with autosomal dominant inheritance was reported by Saldino et al. (1972).
Noack (1959) reported a 43-year-old man and his 11-month-old daughter, both of whom exhibited acrocephaly and polysyndactyly. Enlarged thumbs and great toes with duplication of the latter (preaxial polydactyly) were described, as well as syndactyly. Intelligence was apparently normal. Follow-up of Noack's kindred by Pfeiffer (1964, 1969) indicated that the disorder is the same as acrocephalosyndactyly type V. See HISTORY section.
Baraitser et al. (1980) reported a kindred particularly instructive as to the range of variability. The proband had the full-blown syndrome, whereas 8 persons in 4 sibships of the previous 3 generations had large halluces and partial syndactyly of the toes (mainly toes 2 and 3). The variability of expression was also illustrated by Vanek and Losan (1982). Kroczek et al. (1986) described Kleblattschaedel in association with Pfeiffer syndrome. Rasmussen and Frias (1988) described a girl with severe manifestations of Pfeiffer syndrome. The case was thought to represent a new mutation until the mother was examined in detail and found to show abnormalities of the right thumb consistent with mild expression of the Pfeiffer syndrome. The mother was thought to have mild midfacial hypoplasia. The possibility of mosaicism in the mother seems strong. The mother's father was 40 years old at the time of her birth.
Stone et al. (1990) described an infant with the Pfeiffer syndrome in whom the trachea showed replacement of the cartilaginous rings by a solid cartilaginous plate extending the full length of the trachea and beyond the carina. This resulted in tracheal stenosis.
Soekarman et al. (1992) described classic Pfeiffer syndrome in mother and son. The infant son had cloverleaf skull anomaly. The development in the child after surgery appeared to be normal, indicating that all children with the cloverleaf skull abnormality do not have a dire prognosis.
Vallino-Napoli (1996) reviewed the audiologic and otologic features of 9 patients with Pfeiffer syndrome, ranging in age from 2 to 12 years. Hearing loss was found in 8 of the 9 patients. The degree of loss varied but was moderate to severe in most patients. Seven patients had conductive hearing loss and 1 had mixed loss; none had purely sensorineural loss. Four patients had a history of middle ear effusion. Primary CT findings consisted of stenosis and/or atresia of the external auditory canal, hypoplasia of the middle ear cavity, and an enlarged middle ear cavity. The ossicles were hypoplastic in a few cases. With 1 exception, inner ear anatomy was normal.
Robin et al. (1998) reported the clinical course of 7 children with Pfeiffer syndrome type 3. Although all of them had severe manifestations, development was essentially normal in 3, mildly delayed in 2, and moderately delayed in 1.
Craniofacial-Skeletal-Dermatologic Dysplasia
Shotelersuk et al. (2002) described a 15-year-old Thai boy with an unspecified craniosynostosis syndrome characterized by multiple suture craniosynostoses, a persistent anterior fontanel, corneal scleralization, choanal stenosis, atresia of the auditory meatus, broad thumbs and great toes, severe scoliosis, acanthosis nigricans, hydrocephalus, and mental retardation. Radiography revealed bony ankyloses of vertebral bodies at T9-T12 as well as ankyloses of humeral-radial-ulnar joints, intercarpal joints, distal interphalangeal joints of the fifth fingers, fibulo-tibial joints, intertarsal joints, and distal interphalangeal joints of the first toes.
DiagnosisPrenatal Diagnosis
Gonzales et al. (2005) reported 3 fetuses diagnosed prenatally with severe Pfeiffer syndrome, who all had the same heterozygous mutation in the FGFR2 gene (S351C; 176943.0024). All 3 patients had a cartilaginous tracheal sleeve at autopsy with no visible tracheal rings. In addition, all had vertebral anomalies, including cervical, thoracic, and lumbar fusion, and sacrococcygeal eversion was also present in 2 cases.
InheritancePfeiffer syndrome is an autosomal dominant disorder (Pfeiffer, 1964; Saldino et al., 1972). Cohen (1993) stated that 7 Pfeiffer syndrome pedigrees (three 3-generation and four 2-generation) had been reported, in addition to at least a dozen sporadic cases.
In a study of sporadic cases of Crouzon syndrome and Pfeiffer syndrome, Glaser et al. (2000) used 4 intragenic polymorphisms to screen a total of 41 families. Of these, 22 (11 for each syndrome) were informative. They found 11 different mutations in the 22 families. By molecular means they proved that the origin of these different mutations was paternal in all informative cases analyzed. Advanced paternal age was noted for the fathers of patients with Crouzon syndrome or Pfeiffer syndrome, compared with the fathers of control individuals (34.50 +/- 7.65 years vs 30.45 +/- 1.28 years, P less than 0.01). The data extended previous information on advanced paternal age for sporadic FGFR2 mutations causing Apert syndrome and FGFR3 mutations causing achondroplasia.
MappingChromosome 8
Robin et al. (1994) demonstrated linkage of markers from chromosome 8 in some Pfeiffer syndrome families. By performing fluorescence in situ hybridization on artificial chromosomes (YACs) that contained the linked DNA markers, they localized 1 gene for Pfeiffer syndrome to the pericentromeric region of chromosome 8. Genetic heterogeneity in the syndrome was demonstrated by exclusion of close linkage in other families. Because FGFR1 had been mapped to 8p12-p11.2, it became a strong candidate gene for Pfeiffer syndrome.
Chromosome 10
By linkage analysis in families with Pfeiffer syndrome unlinked to chromosome 8, including the family originally reported by Pfeiffer (1964), Schell et al. (1995) found linkage to chromosome 10q between markers D10S190 and D10S587. Crouzon syndrome (123500) had previously been linked to this region and to be caused by mutation in the FGFR2 gene.
Molecular GeneticsPfeiffer Syndrome with Mutations in the FGFR1 Gene
Muenke et al. (1994) identified a specific mutation in the FGFR1 gene (P252R; 136350.0001) in all affected members of 5 unrelated Pfeiffer syndrome families linked to chromosome 8.
Rossi et al. (2003) reported 4 affected families with the common FGFR1 P252R mutation, all of whom demonstrated characteristic malformation of the feet but variable or absent skull involvement. The feet have the appearance of a broad and flattened hallux that is usually medially deviated, and there is syndactyly of the second and third toes. The authors suggested that this characteristic appearance of the feet, even in an isolated case without craniosynostosis, should prompt a search for the P252R mutation in FGFR1.
Pfeiffer Syndrome with Mutations in the FGFR2 Gene
Schell et al. (1995) identified mutations in the FGFR2 gene in patients with Pfeiffer syndrome linked to chromosome 10. Lajeunie et al. (1995) and Rutland et al. (1995) identified mutations in the FGFR2 gene in some patients with Pfeiffer syndrome. Lajeunie et al. (1995) described FGFR2 mutations in 1 sporadic case and 1 familial form of Pfeiffer syndrome. Rutland et al. (1995) reported point mutations in FGFR2 in 7 sporadic Pfeiffer syndrome patients; 6 of these patients shared 2 missense mutations that had also been reported in Crouzon syndrome (see 176943.0001 and 176943.0002). The Crouzon and Pfeiffer phenotypes usually 'breed true' within families and the finding of identical mutations in unrelated individuals giving different phenotypes was a highly unexpected observation.
Bellus et al. (1996) described a pro250-to-arg mutation in the extracellular domain of the FGFR3 gene (134934.0014) in 10 unrelated families with dominant craniosynostosis syndromes. This mutation (749C-G) occurs precisely at the position in FGFR3 analogous to that of mutations in FGFR1 (P252R; 136350.0001) and FGFR2 (P253R; 176943.0011) reported in Pfeiffer syndrome and Apert syndrome, respectively. The FGFR mutations in Pfeiffer syndrome and nonsyndromic craniosynostosis were reviewed in detail.
In a patient with severe Pfeiffer phenotype, Tartaglia et al. (1997) reported a de novo G-to-C transversion in exon IIIa of the FGFR2 gene, resulting in a trp-to-cys missense mutation at codon 290 (T290C; 176943.0019). The patient had cloverleaf skull deformity as well as the other typical ocular, hand, and foot anomalies seen in Pfeiffer syndrome. Missense mutations at codon 290 of FGFR2 had been reported previously in Crouzon syndrome, but not in Pfeiffer syndrome.
Schaefer et al. (1998) likewise found a T290C mutation in a case of Pfeiffer syndrome type 2 (176943.0032). The infant had cloverleaf skull, proptosis, radioulnar synostosis, and broad thumbs and great toes; however, there were many overlapping findings with Antley-Bixler syndrome (207410). Trp290 appears to be a major hotspot in the FGFR2 gene; a trp290-to-arg substitution was found by Meyers et al. (1996) in classic cases of Crouzon syndrome, and a trp290-to-gly mutation was found by Park et al. (1995) in an atypically mild form of Crouzon syndrome. Schaefer et al. (1998) pictured (their Fig. 2) the sequence in the region of the 2 FGFR2 hotspots, trp290 and cys342, defining the immunoglobulin-like domain of exons 7 and 9.
Plomp et al. (1998) reported 5 unrelated patients, 3 boys and 2 girls, with Pfeiffer syndrome type 2. Most patients with this form died shortly after birth. Causes of death included pulmonary problems, brain abnormalities, prematurity, and postoperative complications. Two of the patients showed the cys342-to-arg mutation (176943.0002).
Craniofacial-Skeletal-Dermatologic Dysplasia
In a 15-year-old Thai boy with a craniofacial-skeletal-dermatologic dysplasia, Shotelersuk et al. (2002) identified heterozygosity for an 870G-T transversion in the FGFR2 gene, resulting in a W290C substitution in the extracellular domain of the protein gene (176943.0032). The same mutation had been reported by Schaefer et al. (1998) in a case of Pfeiffer syndrome with overlapping features of Antley-Bixler syndrome (207410).
HistoryThe disorder described by Noack (1959) was listed in an early edition of MIM as 'acrocephalopolysyndactyly type I (ACPS I)' and was thought to differ from Apert syndrome (101200) by the presence of polydactyly as an additional feature. In this early classification, Carpenter syndrome (201000) was designated 'acrocephalopolysyndactyly II (ACPS II).'
Robinow and Sorauf (1975) described an extensively affected kindred with what they called 'Noack syndrome.' The disorder in the family reported by Robinow and Sorauf (1975) is discussed in 180750.