Classic Hairy Cell Leukemia
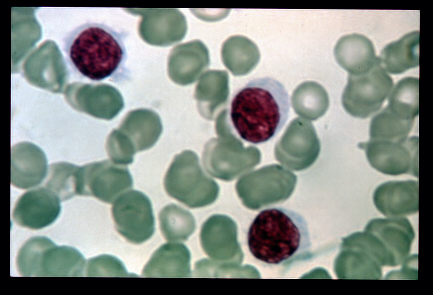
A rare, slowly progressive, chronic leukemia characterized by presence of abnormal B-lymphocytes (medium sized with abundant irregular pale cytoplasm, hair-like cytoplasmic projections/ruffled cytoplasmic border, a round or bean-shaped nucleus and absent nucleoli) in the blood or bone marrow, spleen and peripheral blood pancytopenia, notable monocytopenia, and marked susceptibility to infection. The characteristic immunophenotype is CD11c+, CD25+, CD103+ and CD123+ with a BRAF mutation in most cases.
Epidemiology
Classic hairy cell leukemia (HCLc) accounts for 2% of all leukemia cases. Estimates of the annual incidence range between 1/213,000-2,860,000 worldwide. HCLc is observed more commonly in caucasians. Men are predominantly affected with a male:female ratio of 4:1.
Clinical description
Disease onset is typically in middle-age or older adults with an average age of 55 years. Symptoms of HCLc are related to the disruption of normal blood cell production. Low red cell production leads to anemia, low white cell production to increased infections, and low platelet counts to bleeding or easy bruising. Abdominal discomfort is a common symptom, resulting from hepatosplenomegaly. Splenomegaly is present in most cases, and was originally classed as massive in more than 80% of cases. In recent years, massive splenomegaly is less frequent perhaps related to earlier diagnosis. Hepatomegaly with mild liver dysfunction is found in 20% of cases and lymphadenopathy is found in 10% of cases. Complications include recurrent infections, bleeding, bruising, anemia and abdominal discomfort from splenomegaly. Splenic rupture may occur. Patients with HCLc often have monocytopenia. The patients may have opportunistic infections as a result of being immunocompromised due to intrinsic disease-related immune deficiency as well as immunosuppressive treatment.
Etiology
Etiology is unknown. Family history of blood cancers, Ashkenazi Jewish heritage, occupational or environmental exposure to chemicals (e.g. insecticides) are considered as possible risk factors. BRAFV600E mutation, which causes constitutive activation of the MAP kinase pathway, is also found in most patients with HCLc.
Diagnostic methods
Diagnosis is based on the results of the physical examination, blood tests, and bone marrow biopsy. Abdominal computer tomography may also identify lymphadenopathy. Immunophenotypic evaluations of peripheral blood or bone marrow are essential for establishing the diagnosis. Characteristic immunophenotypic markers include CD11c, CD25, CD103, CD123 positive. BRAF-V600E mutation is also found in most patients.
Differential diagnosis
Differential diagnoses includes hairy cell leukemia variant, splenic marginal zone lymphoma, and splenic diffuse red pulp B-cell lymphoma.
Management and treatment
HCLc can be treated with chemotherapy (cladribine or pentostatin) or biological therapy (interferon alpha, rituximab). The purine nucleoside analogs, either cladribine (indication authorized in Europe and the USA) or pentostatin, have a higher rate of complete remission compared to biological therapies. Complete or partial remission with chemotherapy is achieved in about 80 to 90% of patients. In patients with active infection, it is important to attempt to control infection before using cladribine because of potential profound and prolonged myelosuppression. Treating patients with uncontrolled infection is challenging. Vermurafenib has been used as a bridge to effective therapy in some patients with life-threatening, uncontrolled infection and proven BRAF-positive mutation. These patients have had anecdotal improvement in hematologic parameters with control of infection; however, more evidence is required. Off-label usage of vemurafenib has been reported to be effective in hairy cell leukemia, in particular it has helped achieve remission in patients with relapsed or resistant disease. Currently, studies are in progress to examine the use of this agent in combination chemoimmunotherapy. For patients with hairy cell leukemia who have a relapse following a prolonged period of initial remission, they may be successfully re-treated with a purine analog or a combination of the purine analog and an anti-CD20 monoclonal antibody (rituximab). For those patients who have had multiple relapses or disease refractory to standard therapy, the anti-CD22 immunotoxin conjugate, moxetumomab (approved in the USA), may also be considered. Where the disease is mild and slow growing, a small number of patients may not require any immediate treatment and remain stable for many years. However, close follow-up of the hematologic parameters is required to avoid the development of serious pancytopenia.
Prognosis
Most patients live 10 years or longer with the disease.