Arginase Deficiency
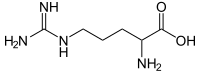
Summary
Clinical characteristics.
Arginase deficiency in untreated individuals is characterized by episodic hyperammonemia of variable degree that is infrequently severe enough to be life threatening or to cause death. Most commonly, birth and early childhood are normal. Untreated individuals have slowing of linear growth at age one to three years, followed by development of spasticity, plateauing of cognitive development, and subsequent loss of developmental milestones. If untreated, arginase deficiency usually progresses to severe spasticity, loss of ambulation, complete loss of bowel and bladder control, and severe intellectual disability. Seizures are common and are usually controlled easily. Individuals treated from birth, either as a result of newborn screening or having an affected older sib, appear to have minimal symptoms.
Diagnosis/testing.
The diagnosis of arginase deficiency is established in a proband with suggestive clinical and/or biochemical findings and confirmed by identification of biallelic pathogenic variants in ARG1 or, in limited instances, by failure to detect arginase enzyme activity (usually <1% of normal) in red blood cell extracts.
Management.
Treatment of manifestations: Management should closely mirror that for urea cycle disorders, except that individuals with arginase deficiency are not as likely to have episodes of hyperammonemia; if present, such episodes respond to conservative management (e.g., intravenous fluid administration). Treatment should involve a team coordinated by a metabolic specialist. Routine outpatient management includes restriction of dietary protein and consideration of oral nitrogen-scavenging drugs (in those who have chronic or recurrent hyperammonemia). Treatment of an acutely ill (comatose and encephalopathic) individual requires: rapid reduction of plasma ammonia concentration; use of pharmacologic agents (sodium benzoate and/or sodium phenylbutyrate/phenylacetate) to promote excretion of excess nitrogen through alternative pathways; and introduction of calories supplied by carbohydrates and fat to reduce catabolism and the amount of excess nitrogen in the diet while avoiding overhydration and resulting cerebral edema. Standard treatment for seizures, spasticity, developmental delay / intellectual disability, and joint contractures. In those with persistent hepatic synthetic function abnormalities, fresh-frozen plasma should be considered prior to surgical procedures. In the rare instance of progression to hepatic fibrosis and cirrhosis, liver transplantation can be considered.
Prevention of primary manifestations: Maintenance of plasma arginine concentration as near normal as possible through restriction of dietary protein and use of oral nitrogen-scavenging drugs as necessary to treat hyperammonemia. Liver transplantation eliminates hyperargininemia and presumably the risk for hyperammonemia but is rarely necessary in arginase deficiency.
Surveillance: Regular follow up at intervals determined by age and degree of metabolic stability. Assessment of metabolic control (plasma ammonia, amino acid profile, and nutritional labs) at least monthly for the first year of life and as determined by a metabolic specialist after the first year of life; guanidinoacetate and liver function tests every six to 12 months; monitoring of growth and developmental progress at each visit.
Agents/circumstances to avoid: Valproic acid (exacerbates hyperammonemia).
Evaluation of relatives at risk: Plasma quantitative amino acid analysis, molecular genetic testing (if the family-specific pathogenic variants are known), or enzymatic testing in all sibs (especially younger ones) of a proband to allow early diagnosis and treatment of those found to be affected.
Pregnancy management: In general, affected pregnant women should continue dietary protein restriction and ammonia-scavenging medications (after an appropriate benefit/risk calculation) based on their clinical course before pregnancy.
Other: Immunizations on the usual schedule; appropriate use of antipyretics as indicated (ibuprofen preferred over acetaminophen).
Genetic counseling.
Arginase deficiency is inherited in an autosomal recessive manner. At conception, each sib of an affected individual has a 25% chance of being affected, a 50% chance of being an asymptomatic carrier, and a 25% chance of being unaffected and not a carrier. Heterozygotes (carriers) are asymptomatic. Carrier testing for at-risk relatives and prenatal testing for pregnancies at increased risk are possible if the ARG1 pathogenic variants in the family are known.
Diagnosis
Suggestive Findings
Scenario 1. Abnormal newborn screening (NBS) result
- NBS for arginase deficiency is primarily based on quantification of the analyte arginine on dried blood spots.
- Arginine values above the cutoff reported by the screening laboratory are considered positive and require follow-up biochemical testing (see Preliminary laboratory findings below).
- If these studies support the diagnosis of arginase deficiency, additional testing is required to establish the diagnosis (see Establishing the Diagnosis).
Note: (1) Some infants with arginase deficiency may have follow-up arginine levels in the normal range, and thus infants who continue to have elevated arginine-to-ornithine ratios and arginine toward the upper limit of normal should undergo additional diagnostic testing (see Establishing the Diagnosis) [Author, personal observation]. (2) Arginase deficiency is currently a secondary condition on the Recommended Uniform Screening Panel. Thus, not all states will screen for and detect newborns with arginase deficiency.
Scenario 2. Symptomatic individual with either atypical findings or untreated arginase deficiency resulting from any of the following:
- NBS not performed
- False negative NBS result
- Caregivers not compliant with recommended treatment following a positive NBS result
Supportive but nonspecific clinical findings and preliminary laboratory findings can include the following.
Clinical findings
- Slowing of linear growth at age one to three years
- Development of spasticity in the lower extremities
- Plateauing of cognitive development
- Loss of developmental milestones
- Seizures
Preliminary laboratory findings
- Plasma quantitative amino acid analysis. Elevation of plasma arginine concentration three- to fourfold the upper limit of normal is highly suggestive of the diagnosis. Plasma arginine elevation is the primary means of ascertainment.Note: Up to twofold the upper limit of normal may be seen in infants who do not have arginase deficiency and who are otherwise normal.
- Plasma ammonia concentration. Elevation of plasma ammonia concentration may be intermittent. Acute hyperammonemia (plasma ammonia concentration >150 µmol/L) is uncommon.
- Urinary orotic acid concentration. Although urinary orotic acid concentration is often elevated, it is not a primary screen for this disorder.
Note: Because elevations of these metabolites individually are not entirely specific to arginase deficiency, follow-up testing is required to establish or rule out the diagnosis of arginase deficiency (see Establishing the Diagnosis).
Establishing the Diagnosis
The diagnosis of arginase deficiency is established in a proband with suggestive clinical and/or biochemical findings and confirmed by identification of biallelic pathogenic variants in ARG1 (see Table 1) or, in limited instances, by failure to detect arginase enzyme activity (usually <1% of normal) in red blood cell extracts. Because of its relatively high sensitivity, ARG1 molecular genetic testing is the preferred confirmatory test for arginase deficiency.
Note: Enzyme assay can be helpful if two pathogenic variants are not found on molecular genetic testing.
Molecular Genetic Testing Approaches
Scenario 1. Abnormal newborn screening (NBS) result. When NBS results and other laboratory findings suggest the diagnosis of arginase deficiency, molecular genetic testing approaches can include single-gene testing or use of a multigene panel:
- Single-gene testing. Sequence analysis of ARG1 detects small intragenic deletions/insertions and missense, nonsense, and splice site variants; depending on the method used, exon or whole-gene deletions/duplications may not be detected. Perform sequence analysis first. If only one or no pathogenic variant is found, perform gene-targeted deletion/duplication analysis to detect intragenic deletions or duplications.Note: In individuals of French Canadian ancestry, the c.57+1G>A founder variant may be tested for first.
- A multigene panel that includes ARG1 and other genes of interest (see Differential Diagnosis) is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.For an introduction to multigene panels click here. More detailed information for clinicians ordering genetic tests can be found here.
Scenario 2. Symptomatic individual with atypical findings or untreated arginase deficiency (resulting from NBS not performed or false negative NBS result):
- If arginase deficiency is suspected, single-gene testing or a multigene panel may be performed (see Scenario 1).
- When the diagnosis of arginase deficiency has not been considered, comprehensive genomic testing (which does not require the clinician to determine which gene[s] are likely involved) is an option. Exome sequencing is most commonly used; genome sequencing is also possible.For an introduction to comprehensive genomic testing click here. More detailed information for clinicians ordering genomic testing can be found here.
Table 1.
Molecular Genetic Testing Used in Arginase Deficiency
| Gene 1 | Method | Proportion of Pathogenic Variants 2 Detectable by Method |
|---|---|---|
| ARG1 | Sequence analysis 3 | >98% 4 |
| Gene-targeted deletion/duplication analysis 5 | <2% 4, 6 |
- 1.
See Table A. Genes and Databases for chromosome locus and protein.
- 2.
See Molecular Genetics for information on allelic variants detected in this gene.
- 3.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Pathogenic variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 4.
Diez-Fernandez et al [2018]; data derived from Human Gene Mutation Database [Stenson et al 2017]
- 5.
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications.
- 6.
Three single or multiexon deletions have been reported [Korman et al 2004, Wang et al 2012, Diez-Fernandez et al 2018].
Measurement of Red Blood Cell Arginase Enzyme Activity
Most affected individuals have no detectable arginase enzyme activity (usually <1% of normal) in red blood cell extracts.
Note: (1) Although arginase is stable, a control sample should be obtained and treated identically if the cells are to be shipped to a distant site. (2) Liver and red blood cell arginase activity correlate well; therefore, it is not necessary to perform a liver biopsy when enzyme activity can be measured from a blood sample.
Clinical Characteristics
Clinical Description
To date, more than 260 individuals with arginase deficiency have been identified [Uchino et al 1995; De Deyn et al 1997; Crombez & Cederbaum 2005; Schlune et al 2015; Huemer et al 2016; Therrell et al 2017; Diez-Fernandez et al 2018; Chandra et al 2019; Author, personal observation]. The following description of the phenotypic features associated with this condition is based primarily on individuals with severe disease. It should be noted that a phenotypic spectrum exists, and mildly affected individuals exhibit less severe features. Individuals treated from birth (as a result either of newborn screening or of having an affected older sib) appear to have minimal symptoms [Cederbaum et al 2004].
Growth and feeding. Most commonly, growth at birth and through early childhood is normal.
- At age one to three years, linear growth slows and eventually the majority of affected children demonstrate growth deficiency, which persists if arginase deficiency goes untreated.
- Microcephaly is common and is congenital in some cases.
- Feeding issues may develop, leading to inadequate nutrition. Some require a supplemental feeding tube.
Cognitive development. Initially, cognitive development in infancy and early childhood is normal.
- Starting at age one to three years, previously normal cognitive development slows or stops and the child begins to lose developmental milestones.
- If untreated, arginase deficiency usually progresses to severe intellectual disability with accompanying neurologic findings (see Neurologic features below).
- Full scale IQ in adults is in the 70s, and about half are able to live independently, though they experience significant memory and fine motor deficits [Waisbren et al 2016]. Mildly affected individuals and those treated early in life may be able to hold a job.
- Some children are more severely affected cognitively, whereas others have more severe spasticity and secondary joint contractures.
Neurologic features. In untreated individuals, progressive neurologic signs typically include the development of severe spasticity with loss of ambulation and complete loss of bowel and bladder control.
- Spasticity. Between 80% and 90% of affected individuals develop spasticity of the lower extremities [Huemer et al 2016, Chandra et al 2019].
- Spastic diplegia typically appears between ages two and four years and is often misdiagnosed as cerebral palsy.
- Severe spasticity can lead to joint contractures and lordosis.
- Seizures occur in 60%-75% of affected individuals and are usually controlled easily by anti-seizure medication [Huemer et al 2016, Chandra et al 2019]. Generalized tonic-clonic seizures are the most common seizure type.
- Brain imaging often reveals cortical atrophy. Other parts of the nervous system including basal ganglia, cerebellum, medulla, and spinal cord are largely spared [De Deyn et al 1997].
Hyperammonemia. Unlike the other eight primary urea cycle disorders (see Urea Cycle Disorders Overview), arginase deficiency rarely results in elevated plasma ammonia concentration in the newborn period.
- Episodic hyperammonemia of variable degree may occur during illness but is rarely severe enough to be life threatening, although death has been reported.
- Hyperammonemia presents with vomiting, lethargy, and altered mental status but in some cases is asymptomatic and only recognized if blood ammonia is obtained during an acute illness.
- Older individuals may present with postoperative encephalopathy.
Liver disease. Hepatic dysfunction, if present, is usually mild, manifesting as transaminitis, prolonged coagulation time, and in some cases hepatomegaly. Affected individuals typically do not have bleeding problems from prolonged coagulation time. Rarely, neonatal cholestatic jaundice has been reported [Braga et al 1997, Gomes Martins et al 2010], and cirrhosis can occur. Some adults have developed hepatocellular carcinoma.
Other. Some affected females experience symptomatic hyperammonemia during menstrual cycles. These individuals may require abortive therapy (see Management, Prevention of Primary Manifestations).
Prognosis. While data are not available, the vast majority of affected individuals appear to survive and live long (albeit handicapped) lives.
Genotype-Phenotype Correlations
Genotype-phenotype correlations indicate that the amount of residual enzyme activity modulates the phenotype [Diez-Fernandez et al 2018]. Severe disease is associated with:
- Homozygosity or compound heterozygosity for predicted loss-of-function variants such as c.466-2A>G, c.77delA, c.263_266delAGAA, and c.647_648ins32;
- Missense changes such as p.Ile8Lys or p.Gly106Arg when homozygous or in combination with another severe allele.
Prevalence
Arginase deficiency is one of the rarest urea cycle defects. Its incidence has been estimated at between 1:350,000 and 1:1,000,000; the true incidence in nonrelated populations is unknown.
Arginase deficiency is pan ethnic but may be more common among French Canadians due to a pathogenic founder variant [Uchino et al 1995] (see Table 9).
Differential Diagnosis
Hyperammonemia. Arginase is the sixth and final enzyme of the eight known steps in the urea cycle. See Urea Cycle Disorders Overview for approaches to distinguish:
- Other causes of hyperammonemia from a urea cycle disorder; and
- The differences between the urea cycle disorders themselves.
Spasticity. Arginase deficiency may be misdiagnosed as static spastic diplegia (cerebral palsy). See Hereditary Spastic Paraplegia Overview. It should be noted that arginase deficiency is one of the few treatable causes of spastic diplegia [Prasad et al 1997].
ARG2. A second arginase gene is known (ARG2), but no human deficiency state has been identified and it is not clear that elevated plasma arginine would be a part of such a deficiency.
CAT-2. A new metabolic disorder in the human cationic amino acid transporter-2 has been proposed. The biochemical profile includes high levels of arginine, ornithine, and lysine in both blood and urine. The one described affected individual presented with an abnormal newborn screen for arginase deficiency [Yahyaoui et al 2019].
Management
No consensus clinical management guidelines for arginase deficiency have been published. However, general guidelines for the management of urea cycle disorders are available [Häberle et al 2019].
Evaluations Following Initial Diagnosis
To establish the extent of disease and needs in an individual diagnosed with arginase deficiency, the following evaluations summarized in Table 2 (if not performed as part of the evaluation that led to the diagnosis) are recommended.
Table 2.
Recommended Evaluations Following Initial Diagnosis in Individuals with Arginase Deficiency
| Evaluation | Comment |
|---|---|
| Obtain plasma ammonia, amino acid profile, guanidinoacetate, & liver function tests. 1 | Consultation w/metabolic physician / biochemical geneticist |
| Gastroenterology / nutrition / feeding team eval |
|
| Developmental assessment | Consider referral to developmental pediatrician. |
| Neurologic eval | Consider referral to neurologist if spasticity is present or seizures are suspected. |
| Musculoskeletal eval | To assess for secondary joint contractures & lordosis. Consider referral to rehabilitation medicine. |
- 1.
Albumin, bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, prothrombin time (PT), and partial thromboplastin time (PTT).
Treatment of Manifestations
The management of individuals with arginase deficiency should closely mirror that described in the Urea Cycle Disorders Overview, with one caveat: individuals with arginase deficiency are less prone to episodes of hyperammonemia and when present, hyperammonemia is more likely to respond to conservative management such as intravenous fluid administration. However, the individual who is comatose and encephalopathic is at high risk for severe brain damage and should be treated accordingly. Arginine supplementation is obviously contraindicated.
Table 3.
Routine Outpatient Management in Individuals with Arginase Deficiency
| Principle | Treatment | Consideration/Other |
|---|---|---|
| Restriction of dietary protein 1 |
|
|
| Administration of oral nitrogen-scavenging drugs | Sodium benzoate
|
|
- 1.
The goal should be maintenance of plasma arginine concentration as near normal as possible.
- 2.
Häberle et al [2019], Urea Cycle Disorders Consortium
Table 4.
Acute Outpatient Management in Individuals with Arginase Deficiency
| Manifestation/Concern | Treatment | Consideration/Other |
|---|---|---|
| Mildly ↑ catabolism 1 |
| Trial of outpatient treatment at home for 12-48 hrs w/assessments for clinical changes 3 |
| Fever | Administration of antipyretics (acetaminophen, ibuprofen) if temperatures rises >38.5°C | |
| Occasional vomiting | Antiemetics |
- 1.
Fever; vomiting, diarrhea, dehydration
- 2.
Some centers advocate reducing natural protein intake to zero or to 50% of the normal prescribed regimen for short periods (24-48 hours) in the outpatient setting during intercurrent illness.
- 3.
Alterations in mentation/alertness, fever, and enteral feeding tolerance with any new or evolving clinical features should be discussed with the designated center of expertise for inherited metabolic diseases.
Table 5.
Acute Inpatient Management in Individuals with Arginase Deficiency
| Manifestation/Concern | Treatment | Consideration/Other |
|---|---|---|
| Hyperammonemia (mild to moderate) | Increase caloric intake:
| Complete restriction of protein should not exceed 24-48 hrs, as depletion of essential amino acids may result in endogenous protein catabolism & nitrogen release. Transition patients from parenteral to enteral feeds as soon as possible. |
| Hyperammonemia (severe) | Same as above, plus nitrogen scavengers:
| |
| Consider intralipids for additional calories or TPN if affected person is unable to tolerate enteral feeds for > few days. | If affected person is unable to hydrate orally, consider placement of NG tube. Avoid overhydration, which can result in cerebral edema. 2 | |
| Dialysis 3 | It is rare for persons w/arginase deficiency to require dialysis. The ammonia level & clinical status determine need for dialysis. |
TPN = total parenteral nutrition
- 1.
High parenteral glucose plus insulin can be used acutely to diminish catabolism.
- 2.
The duration of cerebral edema correlates with poor neurologic outcome.
- 3.
Treatment of choice to most rapidly decrease serum ammonia concentration. The method employed depends on the affected person's circumstances.
Table 6.
Management of Other Complications in Individuals with Arginase Deficiency
| Manifestation/Concern | Treatment | Consideration/Other |
|---|---|---|
| Seizures | Standard AEDs depending on seizure type 1 | Referral to neurologist |
| Spasticity | Consider a trial of Botox®. | |
| Orthotics, walkers, wheelchairs, & other durable medical equipment | Referral to rehabilitation medicine | |
| Persistent hepatic synthetic function abnormalities 2 |
| Referral to hematologist for severe cases |
| Hepatic fibrosis & cirrhosis | Liver transplantation | This is a rare complication. |
| Joint contractures |
| Referral to orthopedist if severe |
AEDs = antiepileptic drugs; FFP = fresh-frozen plasma
- 1.
Valproic acid should be avoided (see Agents/Circumstances to Avoid)
- 2.
Particularly elevated prothrombin time.
The following information represents typical management recommendations for individuals with developmental delay / intellectual disability in the United States; standard recommendations may vary from country to country.
Developmental Disability / Intellectual Disability Management Issues
Ages 0-3 years. Referral to an early intervention program is recommended for access to occupational, physical, speech, and feeding therapy. In the United States, early intervention is a federally funded program available in all states.
Ages 3-5 years. In the United States, developmental preschool through the local public school district is recommended. Before placement, an evaluation is made to determine needed services and therapies and an individualized education plan (IEP) is developed.
Ages 5-21 years
- In the US, an IEP based on the individual's level of function should be developed by the local public school district. Affected children are permitted to remain in the public school district until age 21.
- Discussion about transition plans including financial, vocation/employment, and medical arrangements should begin at age 12 years. Developmental pediatricians can provide assistance with transition to adulthood.
All ages. Consultation with a developmental pediatrician is recommended to ensure the involvement of appropriate community, state, and educational agencies (US) and to support parents in maximizing quality of life. Some issues to consider:
- Individualized education plan (IEP) services:
- An IEP provides specially designed instruction and related services to children who qualify.
- IEP services will be reviewed annually to determine if any changes are needed.
- As required by special education law, children should be in the least restrictive environment feasible at school and included in general education as much as possible and when appropriate.
- PT, OT, and speech services will be provided in the IEP to the extent that the need affects the child's access to academic material. Beyond that, private supportive therapies based on the affected individual's needs may be considered. Specific recommendations regarding type of therapy can be made by a developmental pediatrician.
- As a child enters teen years, a transition plan should be discussed and incorporated in the IEP. For those receiving IEP services, the public school district is required to provide services until age 21.
- A 504 plan (Section 504: a US federal statute that prohibits discrimination based on disability) can be considered for those who require accommodations or modifications such as front-of-class seating, assistive technology devices, classroom scribes, extra time between classes, modified assignments, and enlarged text.
- Developmental Disabilities Administration (DDA) enrollment is recommended. DDA is a US public agency that provides services and support to qualified individuals. Eligibility differs by state but is typically determined by diagnosis and/or associated cognitive/adaptive disabilities.
- Families with limited income and resources may also qualify for supplemental security income (SSI) for their child with a disability.
Prevention of Primary Manifestations
The treatment goal is maintenance of plasma arginine concentration as near normal as possible through restriction of dietary protein intake, supplementation with arginine-free essential amino acid formula, and use of nitrogen-scavenging drugs as needed to treat hyperammonemia. Liver transplantation eliminates hyperargininemia and presumably the risk for hyperammonemia but (in contrast to other urea cycle disorders) is rarely necessary in arginase deficiency; see also Table 3.
Prevention of Secondary Complications
Table 7.
Prevention of Secondary Manifestations in Individuals with Arginase Deficiency
| Manifestation/ Situation | Prevention | Considerations/Other |
|---|---|---|
| Hyperammonemic episodes | Ongoing education of affected persons & caregivers re natural history, maintenance & emergency treatment, prognosis, & risks of acute encephalopathic crises | Written protocols for maintenance & emergency treatment should be provided to parents, primary care providers/pediatricians, & teachers & school staff. 1, 2 |
| Treatment protocols & provision of emergency letters or cards to incl guidance for care in the event of illness | Emergency letters/cards should be provided summarizing key information & principles of emergency treatment for arginase deficiency & containing contact information for the primary treating metabolic center. | |
| MedicAlert® bracelets/pendants, or car seat stickers | ||
| Adequate supplies of specialized dietary products (protein-free formulas; medication required for maintenance & emergency treatment) should always be maintained at home. | For any planned travel or vacations, consider contacting a center of expertise near the destination prior to travel dates. |
- 1.
Essential information including written treatment protocols should be provided before inpatient emergency treatment may be needed.
- 2.
Parents or local hospitals should immediately inform the designated metabolic center if: (1) temperature is >38.5°C; (2) vomiting/diarrhea or other symptoms of intercurrent illness develop; or (3) new neurologic symptoms occur.
Surveillance
Regular follow up at intervals determined by age and degree of metabolic stability is recommended (see Table 8).
Table 8.
Recommended Surveillance for Individuals with Arginase Deficiency
| Manifestation/Monitoring | Evaluation | Frequency |
|---|---|---|
| Assessment of metabolic control | Plasma ammonia, amino acid profile, & nutritional monitoring labs | At least monthly for 1st yr of life; thereafter per metabolic specialist |
| Guanidinoacetate | Every 6-12 mos | |
| Poor growth | Monitor growth | At each visit |
| Developmental delay | Monitor developmental milestones | At each visit in those age <18 yrs |
| Neuropsychological testing using age-appropriate standardized assessment batteries | As needed | |
| Neurologic deterioration 1 | Neurologic eval | At each visit 2 |
| Persistent hepatic synthetic function abnormalities | Liver function tests 3 | Every 6-12 mos |
| Quality of life | Standardized quality of life assessment tools for affected persons & parents/caregivers | As needed |
- 1.
Developmental stagnation and/or regression; seizures; spasticity; development of joint contractures
- 2.
Referral to neurologist, orthopedist, and/or physical therapist as indicated
- 3.
Albumin, bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, prothrombin time (PT), and partial thromboplastin time (PTT).
Agents/Circumstances to Avoid
Valproic acid should be avoided as it exacerbates hyperammonemia in urea cycle defects and other inborn errors of metabolism [Scaglia & Lee 2006].
Evaluation of Relatives at Risk
Because the age of onset of arginase deficiency is delayed beyond the newborn period and the manifestations can vary, the genetic status of all sibs of a proband (especially the younger ones) should be clarified so that morbidity can be reduced by early diagnosis and treatment in those who are affected. Testing methods can include any one of the following:
- Plasma quantitative amino acid analysis
- Molecular genetic testing (if the family-specific ARG1 pathogenic variants are known)
- Analysis of enzymatic activity in red blood cells
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Pregnancy Management
The authors are not aware of any instance in which pregnancy has been reported in a woman with arginase deficiency.
Prior to and During Pregancy
To achieve metabolic control that will enable normal fetal growth and development, affected pregnant women should generally continue dietary protein restriction and ammonia-scavenging medications (after an appropriate benefit/risk calculation) based on their clinical course before pregnancy.
- Protein restriction during pregnancy is challenging given the complications that commonly arise during pregnancy (i.e., nausea, vomiting, anorexia).
- Due to increased protein and energy requirements in pregnancy and, oftentimes, difficulty with compliance, weekly to every two-week monitoring of plasma amino acids and ammonia is recommended, especially in the first and third trimester, and close monitoring immediately after delivery.
- Plasma amino acid levels can help guide quick adjustments to diet in order to achieve normal plasma amino acid profiles that prevent catabolism and hyperammonemia while allowing for normal fetal growth and development.
Fetal Outcomes
There are no well-controlled epidemiologic studies of the fetal effects of sodium benzoate, phenylacetate, or phenylbutyrate during human pregnancy, although there are several case reports.
Redonnet-Vernhet et al [2000] reported a woman with symptomatic ornithine transcarbamylase (OTC) deficiency who was treated with sodium benzoate during the first 11 weeks of gestation and was subsequently transitioned to sodium phenylbutyrate for the remainder of pregnancy. She delivered a healthy female, who at age two years continued to do well.
Lamb et al [2013] reported another woman with symptomatic OTC who was treated throughout pregnancy with sodium benzoate (4 g/4x/day), sodium phenylbutyrate (2 g/4x/day) and arginine (300 mg/4x/day) who delivered a healthy, unaffected male who was doing well at age six weeks.
Ho et al [2019] are the first to document the use of sodium phenylbutyrate throughout two sequential pregnancies in a woman with HHH syndrome:
- In the first pregnancy sodium phenylbutyrate (5.5 g/4x/day) was used as maintenance therapy. This resulted in the delivery of a healthy female who was noted to have typical growth and development at age five years.
- In the second pregnancy, emergency treatment with Ammonul® (sodium phenylacetate/sodium benzoate) to manage hyperammonemic crisis (ammonia 295 µmol/L) was used in addition to maintenance therapy of sodium phenylbuterate (5 g/4x/day).Although the mother responded well to emergency treatment, the baby experienced intrauterine growth restriction and remained in the NICU due to prematurity and low birth weight. At age two years, the child exhibited speech delay and autism.How severe metabolic decompensation, elevated plasma ornithine, and/or side effects of sodium phenylbutyrate, phenylacetate, and/or benzoate may have contributed to the speech delay and/or autism is not known.
- Ho et al [2019] prefer and recommend the use of sodium benzoate if deemed medically necessary during pregnancy, but did not advise switching maintenance medications during pregnancy
Theoretic Concerns
Sodium benzoate has been reported to lead to malformations and neurotoxicity/nephrotoxicity in zebrafish larvae [Tsay et al 2007]. As a known differentiating agent, sodium phenylbutyrate also functions as a histone deacetylase inhibitor with potential teratogenicity, given its ability to alter gene expression in fetal mice [Di Renzo et al 2007]. Theoretically, the use of benzoate/phenylacetate and in particular sodium phenylbutyrate should be avoided during pregnancy, especially during the first trimester. The use of these medications should be carefully evaluated for each individual (benefit/risk ratio) in consultation with a metabolic genetics specialist.
See MotherToBaby for further information on medication use during pregnancy.
Therapies Under Investigation
A clinical trial for enzyme replacement therapy using pegylated synthetic human arginase I is currently under way (Clinical Trials Identifier NCT03921541).
A variety of genomic therapies are under investigation including mRNA therapy [Asrani et al 2018, Truong et al 2019], ARG1 gene editing [Lee et al 2016, Sin et al 2017], and viral-mediated gene therapy [Cantero et al 2016].
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for information on clinical studies for a wide range of diseases and conditions.
Other
Immunizations can be provided on the usual schedule.
Appropriate use of antipyretics is indicated. Ibuprofen is preferred over acetaminophen.