Legg-Calve-Perthes Disease
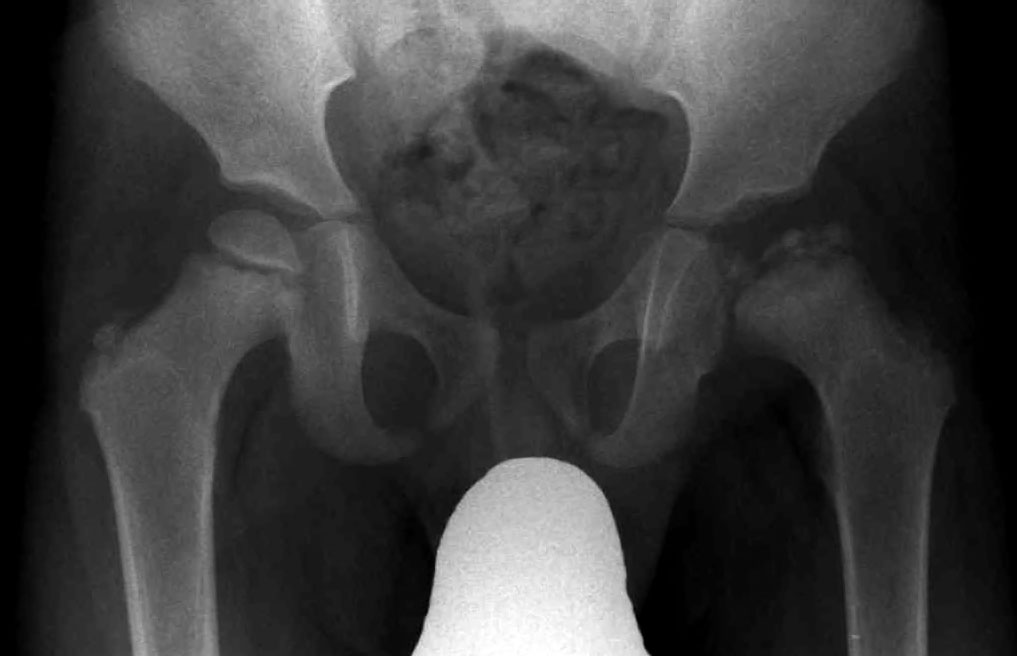
A number sign (#) is used with this entry because of evidence that Legg-Calve-Perthes disease (LCPD), a form of avascular necrosis of the femoral head (ANFH; see 608805) in growing children, is caused by heterozygous mutation in the COL2A1 (120140) on chromosome 12q13.
DescriptionLegg-Calve-Perthes disease is characterized by loss of circulation to the femoral head, resulting in avascular necrosis in a growing child. Clinical pictures of the disease vary, depending on the phase of disease progression through ischemia, revascularization, fracture and collapse, and repair and remodeling of the bone. The disease occurs more frequently in boys, and most patients tend to be shorter than their peers. Both familial and isolated cases of LCPD have been reported (summary by Chen et al., 2004).
Clinical FeaturesWamoscher and Farhi (1963) described a Jewish family in which 8 members of 3 generations were affected. Boys predominate heavily in all reports of sporadic cases of the disease. In the families with multiple cases the sex ratio has been closer to 1. A similar phenomenon has been observed in ankylosing spondylitis (106300) and in congenital dislocation of the hip. When familial, the disorder may be more likely to show bilateral involvement.
McNutt (1962) suggested that a peculiarity in vascular supply of the femoral head and neck may be inherited as the factor predisposing to this disorder. Caffey (1968) was of the view that coxa plana, as he termed this condition, really represents at least in its initiation a stress fracture and not avascular necrosis.
InheritanceStephens and Kerby (1946) observed many affected persons in 5 generations. McKusick (1968) observed affected father and 2 sons, indicating autosomal dominant inheritance.
Gray et al. (1972) found evidence suggesting polygenic inheritance.
Harper et al. (1976) did a population study of Perthes disease in South Wales over a 25-year period. The risk to sibs was less than 1% (2 in 323), and the risk to offspring of an affected parent was about 3% (1 in 35). No increased risk was found in relatives of patients with bilateral rather than unilateral disease.
Hall (1986) combined family data from a series of 87 boys and 58 girls with Perthes disease with those of Gray et al. (1972). Proportions of first-, second-, and third-degree relatives affected in relation to the frequency in the general population showed a gradient of 35:4:4:1 consistent with multifactorial inheritance. She calculated a recurrence risk of 2.6% for sibs and offspring.
Other FeaturesGlueck et al. (1994) suggested that a tendency to thrombosis may predispose to Legg-Perthes disease. In 8 patients with this disorder, they found protein C deficiency (176860) in 3 and protein S deficiency (612336) in 1. Another 1 of the 8 patients had hypofibrinolysis.
PathogenesisAvascular necrosis of the femoral head is a debilitating disease that usually leads to destruction of the hip joint in the third to fifth decade of life (Mont and Hungerford, 1995). Like Legg-Perthes disease, the common pathway of pathogenesis of ANFH is thought to involve the interruption of blood circulation to the femoral head, leading to ischemic insult and bone collapse. Legg-Perthes disease results in ANFH in a growing child.
Molecular GeneticsIn affected members of a Japanese family segregating Legg-Calve-Perthes disease, Miyamoto et al. (2007) identified a missense mutation in the COL2A1 gene (120140.0043). The same mutation had previously been identified in patients with ANFH.
HistoryNevelos (1986) traced the nosography of Perthes disease. Although it had been described on clinical grounds, Perthes disease was not really fully recognized and distinguished from the very common bone and joint tuberculosis until after the discovery of x-rays in 1895.
Animal ModelIn dog models, when venous drainage of the surgical neck of the femur is obstructed, avascular necrosis resembling that of Legg-Perthes disease develops (Liu and Ho, 1991).