Acute Promyelocytic Leukemia
An aggressive form of acute myeloid leukemia (AML), characterized by arrest of leukocyte differentiation at the promyelocyte stage, due to a specific chromosomal translocation t(15;17) in myeloid cells, and manifests with easy bruising, hemorrhagic diathesis and fatigue.
Epidemiology
Acute promyelocytic leukemia (APL) accounts for 10% of AML cases and its incidence is estimated to be 1/1,000,000 people in Europe.
Clinical description
APL onset usually occurs in middle-aged adults and very rarely in pediatric patients. Manifestions include fever, fatigue, dizziness, mild cough with expectoration, exercise-induced dyspnea, weight loss or loss of appetite. Easy bruising or hemorrhagic diathesis (epistaxis, gums bleeding, hematuria, petechiae, metrorrhagia) are frequently observed.
Etiology
The vast majority (93-95%) of APL cases are caused by de novo translocation t(15;17)(q22;q21) that results in the fusion of RARA (retinoic acid receptor-alpha (RARalpha); 17q21.1) with the PML (promyelocytic leukemia (PML); 15q24.1) gene. PML and RARalpha are both implicated in normal hematopoiesis. PML possesses growth suppressor and proapoptotic activity and RARalpha is a transcription factor that mediates effect of retinoic acid (RA) at specific response elements and is believed to be important in proliferation and differentiation of promyelocytes into neutrophils. In a minority of cases (<1%), APL may arise from variant translocations including t(11;17)(q23;q21), t(5;17)(q35;q21), t(11;17)(q13;q21), t(3;17)(q26;q21)der(17), del(17)(q21), t(4;17)(q12;q21), der (2)t(2;17)(q32;q21) whereby RARalpha is fused to ZBTB16, NPM1, NUMA1, TBL1XR1, STAT5b, PRKAR1A, FIP1L1 and NABP1 genes respectively. 35% of APL cases express additional chromosomal abnormalities (trisomy 8, the most frequent, and other abnormalities involving chromosomes 1, 3, 9, 7, 11, 20 and 17, as well as mutations in FLT3 (13q12)). 5-7% of APL cases are related to previous chemotherapy and/or radiotherapy.
Diagnostic methods
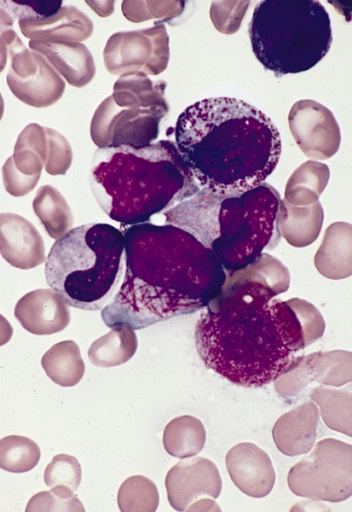
Diagnosis relies on laboratory findings showing pancytopenia (hyperleukocytosis in around 20%) and on bone marrow examination which shows highly granulated promyelocytic cells (scarce granulation if macrogranular), all positive on myeloperoxidase staining. Immunophenotyping of promyelocytic cells shows positivity for CD33, CD13, CD64, CD117, and CD123 and MPO, and negativity for DR, CD34, CD15 and CD65. The diagnosis is confirmed by molecular screening (RT-PCR), anti-PML antibody and cytogenetic or FISH (fluorescence in situ hybridization) analysis of PML/RARA fusion genes.
Differential diagnosis
Differential diagnosis includes the other subtypes of AML.
Management and treatment
The mainstay treatment for APL consists of a combination of either all-trans retinoic acid (ATRA) and anthracycline-based chemotherapy or ATRA and arsenic trioxide (ATO). This latter combination may also be used for relapsed APL and is equally effective as an alternative therapy for elderly APL patients, especially for those considered unfit for chemotherapy. Surveillance for any hemorrhagic signs is necessary, due to the high risk of disseminated intravascular coagulation.
Prognosis
Prognosis of APL after treatment is favorable with complete remission rates of around 90% with 2-year overall survival > 90%. The Sanz score (based on peripheral leukocyte and platelet counts), forms the mainstay for risk stratification in many APL clinical trials; patients with a white blood cell count of > 10 x 109 /L are deemed high risk, with an approximately 15-25% chance of relapse following ATRA + anthracycline chemotherapy. Other factors identified to be associated with an adverse outcome include CD56 expression and advanced age.