Robinow Syndrome, Autosomal Dominant 1
A number sign (#) is used with this entry because of evidence that autosomal dominant Robinow syndrome-1 (DRS1) is caused by heterozygous mutation in the WNT5A gene (164975) on chromosome 3p14.
DescriptionRobinow syndrome, a rare skeletal dysplasia syndrome, is characterized by dysmorphic features resembling a fetal face, mesomelic limb shortening, hypoplastic external genitalia in males, and renal and vertebral anomalies (summary by Roifman et al., 2015).
For a discussion of genetic heterogeneity of Robinow syndrome, see RRS (268310).
Clinical FeaturesRobinow et al. (1969) reported a family with a short stature syndrome inherited over 6 generations. Because of bulging forehead, depressed nasal bridge, and short limbs, achondroplasia (ACH; 100800) was suggested; however, the spine and pelvic radiologic findings were nearly normal. Other features included increased interorbital distance and malaligned teeth. Normal vaginal delivery by affected females was possible. There was no instance of male-to-male transmission. The authors noted similarities to the Aarskog-Scott syndrome (305400); however, the 'saddle scrotum' finding in the Aarskog-Scott syndrome may be the main differentiating feature.
Wadlington et al. (1973) and Vera-Roman (1973) emphasized the occurrence of small or absent penis and hemivertebrae.
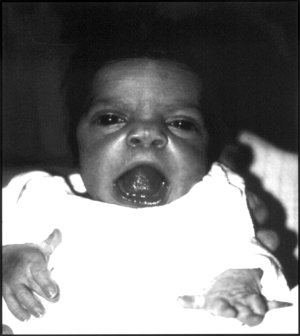
Lee et al. (1982) studied 4 patients with Robinow syndrome. New findings included the following: (1) normal pubertal virilization with persistence of micropenis; (2) elevated follicle-stimulating hormone (see 136530) levels and a hyperresponse of serum luteinizing hormone (see 152780) to gonadorelin stimulation among postpubertal males, suggesting partial primary hypogonadism; (3) normal 5-alpha-reductase (see 607306) and androgen receptor (313700) activity in genital skin fibroblasts; and (4) normal to borderline adult height. Gonadal function and fertility in females seemed to be normal; thus, the lack of male-to-male transmission may be explained. Two of the adult males observed by Lee et al. (1982) were 170 cm and 161.7 cm tall, respectively. During childhood both grew along the 5th percentile for normal boys. The published photograph of the taller of the 2 patients showed acromesomelic brachymelia of the arms, normal virilization, micropenis, and characteristic facies (hypertelorism, midface hypoplasia, and broad mouth).
Friedman (1985) described the distinctive umbilical changes of Aarskog syndrome, Rieger syndrome (180500), and Robinow syndrome. He quoted the famous monograph on the umbilicus by Cullen (1916), which has illustrations by Max Broedel.
Bain et al. (1986) suggested that brachymesomelia is not an essential feature. They reported a father, son, and daughter with typical facial features but normal limbs. The father was 173 cm tall with normal body proportions. He had 'a small but functional penis.' The daughter had a urinary tract infection at age 3 months and was subsequently found to have bilateral renal scarring with grade IV vesicoureteric reflux. The son had the characteristic 'fetal' facies, micropenis, right undescended testis, and a dislocatable right hip. The father apparently represented a new mutation; at the time he was conceived, his father was 52 years of age.
Israel and Johnson (1988) developed a craniofacial profile using 8 angular measurements to study 4 family members with the Robinow syndrome. The profiles showed a high correlation coefficient among affected sibs, a particularly striking finding since most sib correlations in normal families are low by these measurements.
Butler and Wadlington (1987) reported 2 patients, gave a 13-year follow-up on 3 previously reported cases, and reviewed 32 cases in the literature. Autosomal dominant inheritance was reported in 8 persons from 3 families, with male-to-male transmission in 1 family; autosomal recessive inheritance was suggested by the occurrence of 8 sibs from 4 families. No clinical differences were discerned among the individuals with different inheritance patterns.
Turken et al. (1996) described a 1-month-old boy with fetal face, mesomelic shortening of the limbs, and short urethra with an anterior cystic dilatation (see also 268310). In addition, his external genitalia were unusual: his penis was not visible, and there was a large inguinal hernia on the left. On palpation, there was penile tissue, giving the impression of a 'buried penis.'
Kantaputra et al. (1999) described an 11-year-old Thai boy with characteristics typical of the dominant form of Robinow fetal face syndrome and a newborn Caucasian girl with anomalies typical of the recessive form of the syndrome. The boy had some newly recognized signs, including communicating hydrocephalus, underdeveloped sinuses, short roots of the teeth, narrow and thick-floored pulp chambers, hypoplastic nipples, absent middle phalanges of the second to fifth toes, cone-shaped epiphyses of the second and fourth fingers and fifth toes, single creases of the fourth and fifth fingers, clinodactyly of the third fingers, dysmorphic umbilicus, and shawl scrotum.
Patton and Afzal (2002) compared the clinical features of the autosomal dominant and autosomal recessive forms of Robinow syndrome. The recessive Robinow syndrome tended to be more severe.
Mazzeu et al. (2007) reported detailed clinical features of 37 and 51 patients with recessive and dominant Robinow syndrome, respectively. More than 75% of patients with either form had hypertelorism, large nasal bridge, short upturned nose, midface hypoplasia, mesomelic limb shortening, brachydactyly, clinodactyly, micropenis, and short stature. Hemivertebrae and scoliosis were present in more than 75% of patients with the recessive form, but in less than 25% with the dominant form. Umbilical hernia (32%) and supernumerary teeth (10%) were found exclusively in patients with the dominant form.
Beiraghi et al. (2011) compared the craniofacial and intraoral phenotypes of 9 patients with dominant Robinow syndrome to 3 patients with recessive Robinow syndrome. Although there was overlap, particularly with regard to the most prevalent features such as hypertelorism, short, wide nose, and anteverted nares, the craniofacial dysmorphology was more severe in patients with the recessive disorder. In contrast, intraoral features were more severe in patients with the dominant disorder, and included wide retromolar ridge, alveolar ridge deformation, malocclusion, gingival enlargement, dental crowding, and hypodontia. In both types, facial characteristics became less pronounced in older individuals. Beiraghi et al. (2011) suggested that the differential diagnosis may be enhanced by noting differences in the alveolar ridge deformation pattern and severity of other intraoral characteristics.
InheritanceThere are both dominant and recessive (268310) forms of Robinow syndrome.
Wadlington et al. (1973) described an affected brother and sister with normal, nonconsanguineous parents.
Dominant inheritance was documented by Vallee et al. (1982), who described Robinow syndrome in a mother and son.
Robinow (1991) suggested that the dominant form of 'his' syndrome is probably quite rare. Balci et al. (1991) reported 14 patients, all but 1 of whom were the offspring of consanguineous marriages, and Robinow (1991) quoted Baxova of Bratislava, Czechoslovakia, as suggesting that the condition is not rare in Czechoslovakia, where all cases occurred in the offspring of consanguineous gypsy parents (see Baxova et al., 1989). Robinow (1991) also had reports of recessive cases from Saudi Arabia and Kuwait. In addition, he pointed out that some cases thought to be of the dominant variety are probably cases of omodysplasia of Maroteaux (164745), including the 2 patients reported by Bain et al. (1986).
Molecular GeneticsNoting that Wnt5a-null mice exhibit features of Robinow syndrome and that WNT5A interacts with ROR2 (602337), which is mutated in autosomal recessive Robinow syndrome (268310), Person et al. (2010) analyzed the WNT5A gene in affected members of the family with autosomal dominant Robinow syndrome originally reported by Robinow et al. (1969). They identified a pathogenic heterozygous mutation (C182R; 164975.0001). A different heterozygous mutation in the WNT5A gene (C83S; 164975.0002) was found in an unrelated patient with sporadic occurrence of the disorder. Mutations in the WNT5A gene were not found in 23 additional unrelated patients with a clinical diagnosis of dominant Robinow syndrome, suggesting genetic heterogeneity. Functional expression assays in zebrafish embryos showed that the mutant proteins represented hypomorphic alleles rather than dominant-negative mutations. The findings implicated the WNT5A/ROR2 pathway in human craniofacial, skeletal, and genital development.
In affected members of 3 families with autosomal dominant Robinow syndrome, Roifman et al. (2015) identified 2 different heterozygous missense mutations in the WNT5A gene (Y86C, 164975.0003 and C69Y, 164975.0004). The mutation in the first family was found by whole-exome sequencing. Functional studies of the variants were not performed, but molecular modeling indicated that all 4 mutations found to date, including those reported by Person et al. (2010), occurred on 1 side of the protein.
Animal ModelOishi et al. (2003) found that both Wnt5a-null and Ror2 (602337)-null mice showed dwarfism, facial abnormalities, short limbs and tails, dysplasia of lungs and genitals, and ventricular septal defects. In vitro binding assays revealed that Wnt5a binds to the Ror2 and activates the noncanonical Wnt pathway. The findings indicated that Wnt5a and Ror2 interact physically and functionally, and suggested that Ror2 acts as a receptor for Wnt5a to activate noncanonical Wnt signaling.