Porphyria, Acute Intermittent
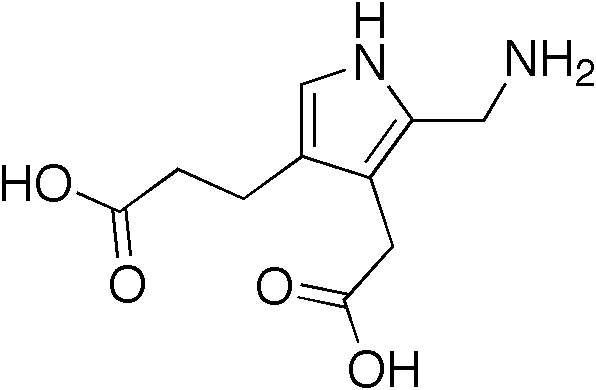
A number sign (#) is used with this entry because acute intermittent porphyria (AIP) is caused by heterozygous mutation in the gene encoding hydroxymethylbilane synthase (HMBS; 609806), also referred to as porphobilinogen deaminase (PBGD), on chromosome 11q23.
DescriptionPorphyrias are inherited defects in the biosynthesis of heme. Acute intermittent porphyria, the most common form of porphyria, is an autosomal dominant disorder characterized by recurrent attacks of abdominal pain, gastrointestinal dysfunction, and neurologic disturbances. In the classic form of AIP, both the ubiquitous 'nonerythroid' housekeeping HMBS isoform and the 'erythroid' HMBS isoform are deficient. However, about 5% of families have the 'nonerythroid variant' of AIP, with a defect only in the ubiquitous nonerythroid HMBS isoform and normal levels of the erythroid HMBS isoform. Clinical characteristics in the 2 forms are identical; diagnostic methods based on the level of enzyme in erythrocytes is ineffective (Puy et al., 1998; Petrides, 1998; Whatley et al., 2000).
There are several other forms of porphyria: see porphyria cutanea tarda (176100), variegata porphyria (176200), coproporphyria (121300), acute hepatic porphyria (125270), and congenital erythropoietic porphyria (263700).
Clinical FeaturesAcute intermittent porphyria is characterized clinically by acute episodes of a variety of gastrointestinal and neuropathic symptoms; between episodes, the patient is healthy. Abdominal pain is the most common symptom, sometimes with constipation and urinary retention; paraesthesias and paralysis also occur, and death may result from respiratory paralysis (Goldberg, 1959; Stein and Tschudy, 1970; Becker and Kramer, 1977). Many other phenomena, including seizures, psychotic episodes, and hypertension, may occur in acute attacks.
Acute attacks rarely occur before puberty; they may be precipitated by porphyrinogenic drugs such as barbiturates and sulfonamides (for list, see Tschudy et al., 1975), some of which are known to induce the earlier rate-controlling step in heme synthesis, delta-aminolevulinic acid (ALA) synthesis. Other known precipitants are alcohol, infection, starvation, and hormonal changes; attacks are more common in women. Only about 10 to 20% of AIP gene carriers become symptomatic during their lifetime (Petrides, 1998).
From a survey of AIP cases in the west of Scotland, Laiwah et al. (1983) observed an association with early-onset chronic renal failure. Porphyria-induced hypertension was considered the most likely causal factor, but enhanced susceptibility to analgesic nephropathy and nephrotoxic effects of porphyrins and their precursors were mentioned as possibilities.
Beukeveld et al. (1990) reported a rare case of a child with presumed homozygous AIP who demonstrated porencephaly and severe developmental retardation. The child consistently excreted excessive amounts of delta-aminolevulinic acid, porphobilinogen, and uroporphyrin in her urine from early childhood. She died at age 8 years. Her mother suffered from AIP. Although the father never had attacks, blood and urine studies showed that he too was affected. Using allele-specific oligonucleotides, Picat et al. (1990) demonstrated that the proband reported by Beukeveld et al. (1990) was compound heterozygous for 2 mutations in the HMBS gene (609806.0005; 609806.0006). Each parent was heterozygous for 1 of the mutations.
Hessels et al. (2004) described a 7-year-old boy with homozygous AIP who demonstrated hepatosplenomegaly, mild anemia, mild mental retardation, yellow-brown teeth, and dark red urine and who had excessively elevated levels of urinary delta-aminolevulinic acid, porphobilinogen, and uroporphyrin. Further hepta-, hexa-, penta- and copro(I)porphyrins were highly increased in urine. This pattern of porphyrin precursor and metabolite excretion is characteristic of acute intermittent porphyria. The porphobilinogen deaminase activity in red cells was decreased to 2 to 4%. The parents were unaffected.
Marsden and Rees (2014) measured urine ALA, PBG, and total urine porphyrin (TUP) excretion in 20 patients with AIP following an attack of acute porphyria for 3 months to 23 years after their last documented acute attack. Urinary concentrations of all metabolites remained elevated for many years. The urinary half life of TUP was 5.3 years, ALA 7.7 years, and PBG 10.6 years. Even after 20 years, PBG concentrations remained elevated above the normal range. Marsden and Rees (2014) concluded that whereas measurement of urine PBG is useful to diagnose the first attack of AIP, it is not helpful as a diagnostic tool in subsequent attacks.
Chester Type Porphyria
McColl et al. (1985) identified a form of acute porphyria in a large family in Chester, U.K. Patients presented with attacks of neurovisceral dysfunction; none had cutaneous photosensitivity. Biochemically, the pattern of excretion of heme precursors varied between individuals. Some had a pattern of acute intermittent porphyria, others showed that of variegate porphyria, and some showed an intermediate pattern. A dual enzyme deficiency was found in peripheral blood cells; reduced activity was found in both PBGD, as in AIP, and protoporphyrinogen oxidase (PPOX; 600923), as in variegate porphyria. McColl et al. (1985) initially thought that this was a new form of porphyria. In the family with Chester type porphyria, Norton et al. (1991, 1993) identified a multipoint maximum lod score of 7.33 at a distance less than 1 cM proximal to D11S351.
In affected members of the original family reported by McColl et al. (1985), Poblete-Gutierrez et al. (2006) identified a heterozygous truncating mutation in the HMBS gene (609806.0046). No mutations were found in the PPOX gene. These findings confirmed that Chester type porphyria is a variant of AIP. Poblete-Gutierrez et al. (2006) suggested that the original biochemical studies indicating PPOX deficiency may have been erroneous or misinterpreted.
Biochemical FeaturesThe essential biochemical finding in acute attacks of AIP is increased urinary excretion of the HMBS precursors delta-aminolevulinic acid (ALA) and porphobilinogen (PBG); this is the basis for the Watson-Schwartz test (Watson and Bossenmaier, 1964). Many latent AIP subjects never have acute attacks, but some intermittently excrete excess porphyrin precursors in urine without having symptoms. Porphyrins may be formed in the urine from the precursors (Waldenstrom, 1956).
Anderson et al. (1979) described abnormalities in steroid metabolism in AIP patients.
Among 22 unrelated families with AIP, Anderson et al. (1981) found differences in the pattern of the 5 stable enzyme-substrate intermediates (A, B, C, D, E) of PBG-deaminase separated by anion-exchange chromatography of erythrocyte lysates. In most patients, the elution profile was similar to the normal with each intermediate reduced about 50%. Some heterozygotes had a second profile in which the C intermediate had disproportionately higher activity than the A or B intermediates; this pattern was observed during an acute attack, suggesting that induction of the enzyme depends on substrate concentrations.
Verma et al. (1987) found that porphyrins are endogenous ligands for a 'peripheral-type' of benzodiazepine receptor, which is selectively associated with the outer mitochondrial membrane. The anxiolytic effects of benzodiazepines are mediated by a 'central' benzodiazepine receptor, located primarily in the brain. The findings may have relevance to the neuropsychiatric aspects of AIP.
Mustajoki and Desnick (1985) used biochemical and immunologic techniques to characterize 4 mutant types of porphobilinogen deaminase in 68 AIP patients from 33 unrelated families in Finland. About 80% of the mutant enzymes were cross-reactive immunologic material (CRM)-negative and fell into 2 types: those in which PBGD levels and enzyme activity were half-normal in all tissues, and a large kindred with normal erythrocyte PBGD levels. The remainder of the families had CRM-positive mutations, including an unusual group that had increased levels of immunoreactive, non-catalytic enzyme. Mustajoki and Desnick (1985) suggested that the CRM-positive patients of the second type have milder disease.
Desnick et al. (1985) further characterized the 4 classes of mutations in AIP by studying 165 AIP heterozygotes from 92 unrelated families. The majority of patients had CRM-negative mutations with half-normal PBGD activity; these were designated 'CRM-negative type 1.' In 3 families, designated 'CRM-negative type 2,' symptomatic patients had increased urinary delta-ALA and PBG with normal levels of erythrocyte PBGD activity. Eleven families had CRM-positive, noncatalytic PBGD. Of these, a subset of patients had increased levels of noncatalytic PBGD with increased levels of substrate-bound intermediates, suggestive of increased binding affinity. The findings indicated allelic heterogeneity for mutations in the PBGD gene.
Nonerythroid Variant of AIP
Mustajoki (1981) reported a large kindred in which 10 members had AIP with normal erythrocyte PBG-deaminase activity. This form is referred to as the 'nonerythroid-variant' of AIP. Forty-nine other Finnish patients with AIP who were unrelated to this kindred had the usual low activity of PBG-deaminase. Mustajoki (1981) suggested that the large kindred represented a variant form of AIP in which the enzyme defect was not expressed in erythrocytes.
In a study of affected members from 22 unrelated families with AIP, Anderson et al. (1981) demonstrated heterogeneity of the erythrocyte porphobilinogen deaminase defect. Although affected members from 21 families had absent CRM to the PBGD protein, all 7 AIP heterozygotes from 1 family of Basque origin had positive CRM results detected in red cell lysates. Further studies showed that this family had a noncatalytic, immunoreactive protein.
Mustajoki and Desnick (1985) and Desnick et al. (1985) also identified patients with AIP who had normal levels and activity of erythrocyte PBG-deaminase.
InheritanceAcute intermittent porphyria, and several other genetic porphyrias, are unusual among enzyme deficiency states in that symptoms are manifest in the heterozygous state, consistent with autosomal dominant inheritance (Meyer and Schmid, 1978).
Although AIP is almost always inherited as an autosomal dominant trait, there have been rare cases of patients with homozygous mutations, consistent with autosomal recessive inheritance. These patients have severe disease and early death (Beukeveld et al., 1990; Llewellyn et al., 1992; Solis et al., 2004).
Doss (1989) described 4 unrelated individuals with coexistent AIP, caused by deficiency of PBGD, and porphyria cutanea tarda (176100), caused by deficiency of uroporphyrinogen decarboxylase (UROD; 613521). The patients manifested clinical courses of both diseases. Family studies showed that in 1 case the dually affected father transmitted both deficiencies to 1 son and only 1 deficiency to a second son. The findings were consistent with the nonlinkage and nonallelism of the 2 genes underlying the disorders.
PathogenesisMustajoki and Desnick (1985) provided a useful illustration of the putative mutation sites in the heme-synthesis pathway in each of 6 forms of porphyria.
In patients with AIP, porphobilinogen deaminase activity reduced to approximately half the normal level was demonstrated first in liver (Strand et al., 1970), and subsequently in cultured fibroblasts and red blood cells (Meyer et al., 1972; Strand et al., 1972; Sassa et al., 1974; Kreimer-Birnbaum, 1975). In family studies, most individuals can be characterized as having either clearly normal or 50% decreased levels of PBGD activity, but intermediate values are sometimes found (Sassa et al., 1974; Astrup, 1978; Kreimer-Birnbaum et al., 1980).
Goldberg (1985) wrote that allelic heterogeneity in AIP may be a factor 'among others, such as drugs, diet, and endogenous hormones, which determine whether the latent state in one patient may continue without incident or, in another, may be shattered by a painful and crippling attack.'
The acute attacks in AIP are precipitated by metabolic, hormonal, and environmental factors that induce hepatic 5-aminolevulinate synthase (ALAS1; 125290) activity. With increased ALAS1 activity, porphyrin precursors ALA and PBG increase, and the half-normal hepatic HMBS activity in heterozygous AIP patients is insufficient to prevent pathologic accumulation of the precursors, which are most likely responsible for the symptoms (Strand et al., 1970; Solis et al., 2004).
Solis et al. (2004) reported a Spanish child with homozygous AIP and a severe clinical course with developmental retardation and early death. Neurologic and neuroradiologic findings suggested a primary process affecting deep cerebral white matter myelination, with relative preservation of the corpus callosum, anterior limb of the internal capsule, cerebral gray matter, brainstem, and cerebellum. Thus, the process affected tracts that myelinate in the later postnatal period, but spared the many tracts that myelinate prenatally or shortly after birth. The selective white matter damage was associated with arrest of myelin maturation at the 8- to 10-month milestones, and later with progressive vacuolation/caviation in the periventricular white matter. The findings were consistent with ALA-mediated neurotoxicity which is manifest postnatally. Solis et al. (2004) noted that if heme deficiency was important in pathogenesis, then it would be expected to cause earlier neuropathologic features. Solis et al. (2004) concluded that the neuropathologic features in this case resulted from postnatal toxic injury, presumably due to the persistent elevation of ALA, PBG, and/or other porphyrin precursors. The findings indicated that the toxic accumulation of porphyrin precursors, rather than heme deficiency, is primarily responsible for acute neurologic attacks in heterozygous AIP.
In their patient, a 7-year-old boy with excessively elevated levels of urinary delta-aminolevulinic acid, porphobilinogen, and uroporphyrin, Hessels et al. (2004) found a novel homozygous leu81-to-pro (L81P) mutation in exon 6 of the porphobilinogen deaminase gene (609806.0045). The father and mother, shown to be gene carriers of the same mutation, are asymptomatic and have normal urinary porphyrin precursor metabolite excretion.
MappingIn 33 unrelated patients with acute intermittent porphyria, Llewellyn et al. (1987) found linkage to a common 2-allele MspI RFLP of the PBGD gene (maximum lod score of 3.14 with no recombinants). In 30 patients, no cross-reacting immunologic material was produced by the mutant allele. A major gene deletion was excluded as the cause of the CRM-negative mutation in 18 heterozygous families.
By PCR, Lee et al. (1990) amplified polymorphic sites in the PBGD gene that could be used for linkage analysis in AIP families.
Scobie et al. (1990) identified three 2-allele RFLPs in the PBGD gene, each with allele frequencies close to 0.50. Marked linkage disequilibrium limited the number of observed haplotypes to 4, of which 1 was uncommon. No common haplotype was observed among 47 unrelated AIP patients.
In 3 Finnish families, each with a different subtype of AIP (CRM-negative with low red cell enzyme activity; CRM-positive with low enzyme activity; CRM-negative with normal enzyme activity), Kauppinen et al. (1990) found evidence of tight linkage to PBGD RFLPs. Among 62 family members tested, 30 had a familial disease-associated haplotype; in 5 of them, conventional tests for AIP were normal, and in 1, uncertain. The authors concluded that RFLP analysis could be used to detect gene carriers and to help in the diagnosis of persons with uncertain results in other tests.
Molecular GeneticsIn a large Dutch family with the nonerythroid variant of AIP, Grandchamp et al. (1989) identified a heterozygous splice site mutation in intron 1 of the PBGD gene (609806.0001). The mutation interrupted the sequence coding for the nonerythroid isoform of PBGD; thus, expression of the erythroid isoform was unaffected. In a patient with CRM-positive AIP, Grandchamp et al. (1989) identified a mutation in the HMBS gene, resulting in the skipping of exon 12 (609806.0002).
In affected members of 11 different families with either CRM-negative or CRM-positive AIP, Grandchamp et al. (1990) identified 7 different point mutations in the PBGD gene.
Astrin and Desnick (1994) reviewed the 26 mutations in the HMBS identified to that time.
Puy et al. (1997) performed molecular analysis of the PBGD gene by denaturing gradient gel electrophoresis followed by direct sequencing in 405 subjects from 121 unrelated French-Caucasian AIP families. PBGD mutations were identified in 109 families, but only 78 were of different type, and each of these had a prevalence rate of less than 5%. Among these mutations, 33 had not previously been published. Sixty percent of the 78 mutations were located in 3 exons (exons 10, 12, and 14); 44% were missense, 18% were splice defect, 19% were frameshift, and 16% were nonsense.
Whatley et al. (1999) reported a prospective comparison of direct automated sequencing of cDNA (in 29 patients) or genomic DNA (in 28 patients) to identify HMBS mutations in 57 patients referred consecutively for mutation analysis; 39 different mutations were identified in 54 patients. The sensitivity of the cDNA and genomic DNA methods was 69% and 95%, respectively, indicating that analysis of genomic DNA provides a higher mutation detection rate. The mutations included 6 missense, 8 splice defects, 10 frameshifts, and 1 nonsense; 25 had not previously been reported. The results defined the extent of allelic heterogeneity and the types (41% missense, 59% truncating) and distribution (35% in exons 10, 12, and 14) of HMBS mutations for AIP in the United Kingdom.
DiagnosisSassa et al. (1975) noted that the enzyme defect in AIP is expressed in cultured fibroblasts and amniotic cells, so that prenatal diagnosis is possible. The enzyme can be induced and the defect demonstrated in mitogen-stimulated lymphocytes (Sassa et al., 1978).
Puy et al. (1997) found that the standard PBGD enzymatic screening method for gene-carrier detection had 95% concordancy with the molecular-based diagnosis.
Clinical ManagementMost acute attacks, if correctly recognized, settle with supportive treatment; dextrose infusion and high carbohydrate intake may be helpful (Stein and Tschudy, 1970). Successful treatment by infusion of hematin, which is a specific feedback inhibitor of heme synthesis, has repeatedly been reported (McColl et al., 1979; Lamon et al., 1979), but hematin is neither readily available nor very soluble and its use may carry a risk of renal damage (Dhar et al., 1978).
Goetsch and Bissell (1986) described a 22-year-old woman who had had more than 15 acute attacks requiring hospitalization. Hematin was the mainstay of the patient's therapy, eliciting a well-defined clinical and biochemical response after more than 200 infusions. On one occasion, however, the patient did not respond to a batch of hematin that, in retrospect, was found to have deteriorated. Goetsch and Bissell (1986) quantified the instability of hematin and showed that the decay product(s) is ineffective in regulating porphyrin production. The decayed material, furthermore, was found to have anticoagulant effects, thus explaining one of the complications of hematin therapy.
An experience reported by Anderson et al. (1984) suggests that in women with premenstrual exacerbation of AIP, a long-acting agonist of luteinizing hormone-releasing hormone may be an effective preventive measure. Srugo et al. (1987) described acute intermittent porphyria as the cause of 'surgical abdomen' in a 15-year-old boy. Because hypertension, tachycardia, and paralytic ileus were present, suggesting sympathetic overactivity, the beta-adrenergic blocking agent propranolol was administered in high doses with apparent dramatic improvement.
In 9 members of a German kindred in which the proband had nonerythroid variant of AIP resulting in a life-threatening coma, Petrides (1998) identified a mutation in the HMBS gene (609806.0001). The newly identified family members were informed of the disease and taught how to prevent porphyric attacks. They were also given medical alert information certificates. Petrides (1998) emphasized the importance of identifying gene carriers as part of the clinical management of AIP.
Soonawalla et al. (2004) reported a 19-year-old woman with severe AIP who underwent successful treatment with liver transplantation. After the transplant, concentrations of heme precursors in the patient's urine returned to normal and 1.5 years later, her quality of life was good.
Pischik and Kauppinen (2015) suggested the following clinical management of AIP. During an acute attack, they recommended treatment with heme preparation if the attack was severe or moderate; symptomatic treatment of autonomic dysfunctions, polyneuropathy, and encephalopathy; exclusion of precipitating factor; and adequate nutrition and fluid therapy. During remissions, they recommended exclusion of precipitating factors, including education of patients and family doctors; information about online drug lists; and mutation screening and education for family members. In patients with recurrent attacks, they recommended evaluation of lifestyle; evaluation of hormonal therapy in women; prophylactic heme therapy; and liver transplantation in patients with severe recurrent attacks. They noted that all AIP patients should be followed up for long-term complications including hypertension, chronic kidney insufficiency, chronic pain syndrome, and hepatocellular carcinoma.
Bissell et al. (2017) reviewed the treatment of patients with AIP. The initial management includes reviewing medications that are considered to be risky for such patients and the administration of fluids (preferably 10% dextrose in 0.45% saline), antiemetic agents, analgesic agents, and, if indicated, antiseizure medications. Antiepileptic drugs must be especially carefully considered. Their Table 3 listed probably safe/possibly unsafe/unsafe medications; unsafe drugs included phenytoin, barbiturates of all type, valproic acid, carbamazepine, and primidone as well as oral contraceptives, progestins, carisoprodol, and spironolactone. The authors cited 2 online resources that provide detailed information on the use of drugs in this disorder. The only specific treatment for acute attacks is intravenous heme (Panhematin in the United States and Normosang in Europe). The authors noted that frequent courses of heme can result in hepatic iron buildup and injury due to iron overload. They cited alternatives to intravenous heme that were being developed and were in clinical trials.
To determine whether chronic hemin administration contributes to the recurrence of acute attacks in AIP, Schmitt et al. (2018) performed a follow-up study of 602 French symptomatic AIP patients, of whom 46 had recurrent AIP, who were seen between 1974 and 2015. The authors also studied the hepatic transcriptome, serum proteome, liver macrophage polarization, and oxidative and inflammatory profiles of Hmbs -/- mice chronically treated by hemin, and extended the investigations to 5 explanted livers from recurrent AIP patients. Schmitt et al. (2018) found that the introduction of hemin into the pharmacopeia coincided with a 4.4-fold increase in the prevalence of chronic patients. Moreover, both in animal model and in human liver, frequent hemin infusions generated a chronic inflammatory hepatic disease which induced HMOX1 (141250) remotely to hemin treatment and maintained a high ALAS1 level responsible for recurrence. Schmitt et al. (2018) suggested that hemin should be restricted to severe neurovisceral crisis only and that alternative treatments targeting the liver, such as ALAS1 and HMOX1 inhibitors, and antiinflammatory therapies should be considered instead of hemin in patients with recurrent AIP.
Sardh et al. (2019) conducted a phase 1 trial of givosiran, an RNA interference therapeutic agent that reduces hepatic ALAS1 mRNA levels, in patients with acute intermittent porphyria. Part A (single injection of an ascending dose) and part B (multiple injections of an ascending dose) were conducted in 23 patients with no attacks in the preceding 6 months. Part C (multiple injections) was conducted in 17 patients who had recurrent attacks. Safety, pharmacokinetic, pharmacodynamic, and exploratory efficacy outcomes were evaluated. Common adverse events included nasopharyngitis, abdominal pain, and diarrhea. Serious adverse events occurred in 6 patients who received givosiran in parts A through C combined. In part C, all 6 patients who were assigned to receive once-monthly injections of givosiran had sustained reductions in ALAS1 mRNA, delta aminolevulinic acid (ALA), and porphobilinogen (PBG) levels to near normal. These reductions were associated with a 79% lower mean annualized attack rate than that observed with placebo. Sardh et al. (2019) concluded that once-monthly injections of givosiran in patients who had recurrent porphyria attacks resulted in mainly low-grade adverse events, reductions in induced ALAS1 mRNA levels, nearly normalized levels of the neurotoxic intermediates ALA and PBG, and a lower attack rate than that observed with placebo.
Population GeneticsHigh prevalence of AIP is known in northern Sweden where Waldenstrom's classic observations were made (Waldenstrom, 1956).
AIP occurs with very low prevalence, perhaps 1 in 50,000, probably in all ethnic groups (Tschudy et al., 1975), including blacks (Kreimer-Birnbaum et al., 1980), but figures for prevalence based on manifest AIP, i.e., acute attacks, greatly underestimate the number of persons with latent AIP.
Lee et al. (1991) stated that the prevalence of AIP in Lappland, northern Sweden was 1 in 1,500. They identified 3 different disease haplotypes among 28 Swedish AIP families. The haplotype designated 2/1/1/2 was the most frequent, segregating with AIP in 10 of 28 families. Lee and Anvret (1991) identified a mutation in the HMBS gene (W198X; 609806.0012) in 15 of 33 AIP families from Lappland, Sweden. Genealogic data showed that 12 of the 15 were related, suggesting a founder effect.
In 28 Finnish families representing 72% of all AIP families in the Finnish population of 5 million, Kauppinen et al. (1995) found 19 separate mutations in HMBS: 13 novel mutations, including 1 de novo event, and 6 previously characterized mutations.
Floderus et al. (2002) stated that the prevalence of AIP in Sweden is about 1 in 10,000. Among Swedish AIP kindreds, they identified 27 novel HMBS mutations, bringing the total number of known mutations in Sweden to 39.
HistoryLoftus and Arnold (1991) suggested that Vincent van Gogh had suffered from attacks of acute intermittent porphyria, exacerbated by malnutrition and absinthe abuse. They suggested that this would best explain the abruptness of the onset and recovery from attacks. It has been suggested that George III had porphyria variegata (176200).
Animal ModelDuring study of the pathogenesis of the neurologic symptoms of AIP, Lindberg et al. (1996) generated Pbgd (HMBS; 609806)-deficient mice by gene targeting. These mice exhibited typical biochemical characteristics of human AIP, including decreased hepatic Pbgd activity, increased delta-aminolevulinic acid synthase activity, and massively increased urinary excretion of the heme precursor delta-aminolevulinic acid after treatment with drugs such as phenobarbital. Behavioral tests revealed decreased motor function and histopathologic findings, including axonal neuropathy and neurologic muscle atrophy.
Puy et al. (1996) noted that a porphyric rat model showed increased plasma concentration and brain uptake of tryptophan and increased synthesis of serotonin in the nervous system. The increased concentration of tryptophan and serotonin may be partly due to the hepatic heme deficiency decreasing the activity of the liver cytosolic enzyme heme-dependent tryptophan pyrrolase. Injection of heme lowered heme precursors, tryptophan, and serotonin to normal levels, but did not increase melatonin, which is produced from tryptophan. Puy et al. (1996) noted that women with AIP demonstrate a rise in serotonin and plasma tryptophan during the attacks, whereas both daytime and nighttime melatonin concentrations are dramatically decreased. From the animal experiments, Puy et al. (1996) suggested that delta-aminolevulinic acid is responsible for decreased production of melatonin by the pineal gland.
Clavero et al. (2010) described a naturally occurring feline model of AIP in 4 unrelated cat lines that presented phenotypically as congenital erythropoietic porphyria (CEP; 263700). Affected cats had erythrodontia, brownish urine, fluorescent bones, and markedly elevated urinary uroporphyrin and coproporphyrin, consistent with CEP. However, their uroporphyrinogen-III synthase (UROS; 606938) activities (deficient in CEP) were normal. Notably, affected cats had half-normal Hmbs activities and elevated urinary 5-aminolevulinic acid and porphobilinogen. Sequencing the feline Hmbs gene revealed different mutations in each line, including a duplication, an in-frame 3-bp deletion (842delGAG), and 2 missense (A84T and R149W) mutations. The 842delGAG and R149W mutations were identical to mutations reported in human. Prokaryotic expression of the 842delGAG and R149W mutations resulted in mutant enzymes with less than 1% wildtype activity, whereas the A84T mutation expressed a stable enzyme with approximately 35% of wildtype activity. The discolored teeth from the affected cats contained markedly elevated URO I and III, accounting for the CEP-like phenocopy. In 3 lines, the phenotype was an autosomal dominant trait, while affected cats with the A84T mutation were homozygous, a unique recessive form of AIP.