Fibrodysplasia Ossificans Progressiva
A number sign (#) is used with this entry because fibrodysplasia ossificans progressiva (FOP) is caused by heterozygous mutation in the ACVR1 gene (102576) on chromosome 2q24.
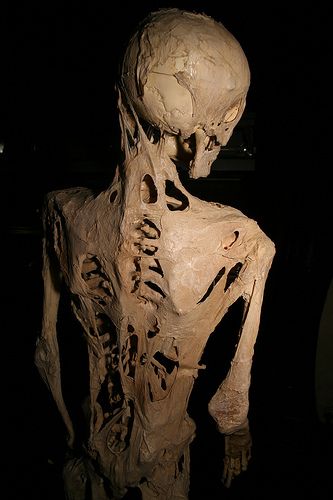
DescriptionFibrodysplasia ossificans progressiva is a rare autosomal dominant disease with complete penetrance involving progressive ossification of skeletal muscle, fascia, tendons, and ligaments. FOP has a prevalence of approximately 1 in 2 million worldwide, and shows no geographic, ethnic, racial, or gender preference. Individuals with FOP appear normal at birth except for great toe abnormalities: the great toes are short, deviated, and monophalangic. Ossification occurs progressively over the course of a lifetime in an inevitable and unpredictable episodic manner, with most patients being confined to a wheelchair by the third decade of life and requiring lifelong care (summary by Petrie et al., 2009).
Clinical FeaturesFibrodysplasia ossificans progressiva is a rare disorder characterized by physical handicap due to intermittently progressive ectopic ossification and malformed big toes which are often monophalangic. Occasional features include short thumbs, fifth finger clinodactyly, malformed cervical vertebrae, short broad femoral necks, deafness, scalp baldness, and mild mental retardation. Although most cases are sporadic, several examples of affected twins and triplets have been reported. Furthermore, dominant inheritance is supported by observations of 2 or 3 successive generations affected and the finding of a paternal age effect in sporadic cases (Tuente et al., 1967). Connor and Evans (1982) found a point prevalence of 0.61 per million in the United Kingdom and gave a direct estimate of the mutation rate of 1.8 per million gametes per generation.
Schroeder and Zasloff (1980) analyzed malformations of the hand and foot in 16 cases of FOP. Connor (1983) provided a comprehensive discussion based on a large personal experience as well as on the literature. Cohen et al. (1993) described the natural history of heterotopic ossification in FOP on the basis of responses to a mail questionnaire. The age of patients at the time of response ranged from 3 to 69 years (average age, 27). The average age at onset of ossification was 5 years (range, birth to 25 years). The most common sites of early heterotopic ossification were the neck, spine, and shoulder girdle. Some restrictive heterotopic ossification was present by age 7 years in 35 of the patients (80%). By the age of 15 years, 42 (more than 95%) of the patients had severely restricted mobility of the arms. Heterotopic ossification proceeded in a direction that was axial to appendicular, cranial to caudad, and proximal to distal.
Connor et al. (1993) reported FOP in 5 persons in 3 successive generations. The affected individual in the first generation was a male. He had 2 affected daughters and 2 affected granddaughters. A wide range of phenotypic severity was demonstrated, from disabling ectopic bone formation and premature death to an asymptomatic adult with characteristic malformations of the big toe. The affected man in the first generation was asymptomatic until he developed back and neck stiffness after trauma. His jaw also became fixed after trauma and by his forties he had developed a limp. With the use of a stick he remained ambulant, however, until his early seventies; he died at 72 years of age from a myocardial infarction. Diagnosis of FOP was confirmed at the Royal National Orthopaedic Hospital, London, where x-rays were preserved. These showed malformed big toes with superimposed ankylosis, progressive ankylosis of the cervical spine, and multiple areas of soft tissue ossification. One of his daughters was asymptomatic until 22 years of age when she developed jaw fixation after extraction of wisdom teeth. Jaw fixation recurred 1 week after replacement of the right temporomandibular joint with a titanium prosthesis. Her sister was well until 15 years of age when spontaneous swelling of the left leg occurred. After biopsy the family was told she had a fibrosarcoma. In her late teens, she developed limitation of movement at the right elbow after trauma. In her twenties, she developed a succession of painless lumps on her back. Thereafter she became gradually more disabled and was bed-bound for a year before her death from pneumonia at 28 years of age. One of the granddaughters had developed painful lumps on the back beginning at the age of 13 years and at age 23 years showed an ectopic bony bar in the left lumbar area. A feature not previously described in FOP was multiple osteochondromata of the hip joints.
Kaplan et al. (1993) documented the transmission of FOP from a sporadically affected father to each of his 3 children, 2 daughters and a son. The father, aged 27 years, was the third son of a 33-year-old mother and a 38-year-old father. He had onset of fibrous nodules in infancy and, at the age of 2 years, heterotopic ossification of the neck developed after blunt trauma. His disorder progressed to severe abnormality, as indicated by the published photographs. Typical deformity of the great toes was present. Characteristic widening of the femoral neck was demonstrated radiographically. All 3 children had malformation of the great toes at birth and subsequently developed typical clinical features. One child developed ossification in the quadriceps after an intramuscular injection in the anterior aspect of the left thigh at the age of 2 months. Another child first developed soft tissue nodules at the age of 3 months.
Janoff et al. (1996) observed classic findings of FOP in 2 Native-American half sisters with the same unaffected mother and different unaffected fathers. The findings were interpreted as suggesting maternal gonadal mosaicism.
Smith et al. (1996) reviewed FOP on the basis of 28 patients studied for up to 24 years. Ossification in the large skeletal muscles began from birth to 16 years (mean age 4.6 years), initially in 25 patients in the neck and upper spinal muscles, and later around the hips, major joints, and jaw. The rate and extent of disability was unrelated to the time of onset. There was no evidence that any form of treatment produced consistent benefit. The initial diagnosis was usually wrong and the mean delay in correct diagnosis after ectopic ossification began was 2.7 years (range 0-14). This delay was mainly due to failure to recognize the significance of the abnormal toes, which were present and potentially recognizable at birth in all cases. Radiographic changes were observable in other bones such as the cervical spine and the metaphyses of the long bones where exostoses were found.
Furuya et al. (2008) described a 62-year-old Japanese man who developed difficulty in moving his shoulders at age 10 years due to contractures of both shoulder joints. The joint contractures progressed slowly in his extremities, and he was unable to walk at age 36 years and bedridden at age 55 years, at which time he had rigid spine, baldness, and sensory hearing loss, accompanied by abnormal ossification but no respiratory failure. He also had severe hypodactyly with short thumbs in both hands and a severe defect of both great toes. Extensive heterotopic bone formation was seen radiographically. His parents died at advanced age with no symptoms reminiscent of FOP, and he had 2 healthy sibs.
Barnett et al. (2011) described a female patient with so-called 'variant FOP' who had normal great toes and late-onset heterotopic ossification and was misdiagnosed with ankylosing spondylitis for several years. The patient presented at 21 years of age with a 'stiff back,' but had a normal x-ray and was unsuccessfully treated with sulfasalazine for a year for 'nonspecific spondyloarthropathy;' several years later she was treated with sulfasalazine and methotrexate for continuing severe back pain that was diagnosed as ankylosing spondylitis, but she discontinued treatment after several months without improvement. Around that time, the patient underwent fine needle aspiration of a painless mass of the mandible that was suggestive of a reactive or inflammatory process in skeletal muscle; the mass resolved completely over 3 months. She later developed right scapular pain that appeared to be myositis of the right rhomboid major, serratus anterior, and intercostal muscles on MRI; this resolved spontaneously over several months. At 27 years of age, upon evaluation of a painless mass in the thyroid region, the patient had plain films and CT scan of the entire spine that revealed extensive ossification of the ligamentum flavum in the thoracic spine and of the interspinous ligament in the lumbar spine, as well as a thin layer of ossification between the right trapezius and rhomboid, consistent with ossification of the previous inflammatory lesion. The diagnosis of FOP was suggested based on the clinical and radiologic findings, and examination revealed significant limitation of neck and lumbar spine movement, particularly flexion and extension, as well as 2 small asymptomatic ossified masses, 1 over the right scapula and 1 over the right lower lumbar spine. Clinical and radiologic examination of the feet was normal; Barnett et al. (2011) stated that this was only the fifth reported FOP patient with bilaterally unaffected great toes.
Hammond et al. (2012) delineated the common facial characteristics of 55 patients with molecularly confirmed FOP by analyzing their 'face signature' (face shape differences normalized against age- and sex-matched controls) and associated face signature graphs. The analysis identified 10 affected individuals whose face signature was more homogeneous than others with FOP; distinctive features included the previously identified reduced mandible and maxillary overbite, as well as low-set ears, underdevelopment of the upper orbit/supraorbital ridge, and infraorbital prominence. Hammond et al. (2012) suggested that the canonical FOP mutation variably affects the postnatal morphogenesis of the normotopic cranial skeleton in the upper midface and mandible.
Severino et al. (2016) performed brain MRI studies in 13 patients with FOP, 11 of whom were carriers of the recurrent R206H mutation in the ACVR1 gene (102576.0001), which is present in the vast majority (95%) of FOP patients; the remaining 2 patients carried the R258S mutation (102576.0003). All 13 patients had small asymptomatic lesions similar to hamartomas in the dorsal medulla and ventral pons, as well as a minor dysmorphism of the brainstem, involving bulging of the dorsal pons, thickened pontomedullary junction, and enlarged medulla. The origin of the vestibulocochlear nerves was enlarged in 10 (77%) of the patients, and facial nerves emerged more anteriorly than normal in all. None of the patients reported any neurologic symptoms, and neurologic examination was normal in all; specifically, there were no deficits of cranial nerves or motor function related to the brainstem lesions and there were no extrapyramidal deficits. The size of the brainstem lesions did not correlate with patient age, age at first flare-up, severity of disability, history of head trauma, or hearing loss. Basal ganglia and/or dentate nuclei calcifications were detected in 8 patients and correlated positively with severity of disability and patient age. Severino et al. (2016) concluded that the effects of ACVR1 mutations extend to the central nervous system, and suggested that these lesions may represent useful disease hallmarks.
Biochemical FeaturesIn a single case, Beratis et al. (1976) found low levels of alkaline phosphatase in cultured skin fibroblasts.
Shafritz et al. (1996) studied bone morphogenetic proteins 1 (112264) to 7 (112267) in lymphoblastoid cell lines and fibroblast-like cell lines from lesional and nonlesional tissue of FOP patients. Among the bone morphogenetic proteins and mRNAs examined, only BMP4 (112262) and its mRNA were present in increased levels in cells derived from an early fibroproliferative lesion in a patient with FOP. BMP4 mRNA was expressed in lymphoblastoid cell lines from 26 of 32 patients with FOP, but from only 1 of 12 normal subjects (P less than 0.001). BMP4 and its mRNA were detected in the lymphoblastoid cell lines from a man with FOP and his 3 affected children (2 girls and a boy), but not from the children's unaffected mother.
In studies of lymphoblastoid cell lines from patients with FOP and controls, de la Pena et al. (2005) found that FOP lymphocytes expressed 6-fold higher levels of type IA BMP receptor (BMPR1A; 601299) on the cell surface compared with control cells and displayed a marked reduction in ligand-stimulated internalization and degradation of BMPR1A. In control cells, BMP4 treatment increased BMPR1A phosphorylation; in FOP cells, BMPR1A was phosphorylated at a high level in the absence of the ligand and showed no increase in response to BMP4. After treatment with the BMP antagonist noggin, BMPR1A phosphorylation decreased in control cells but remained constant in FOP cells, indicating that BMPR1A hyperphosphorylation is independent of ligand stimulation in FOP cells. De la Pena et al. (2005) concluded that altered BMP receptor trafficking may play a significant role in FOP pathogenesis.
DiagnosisKaplan et al. (1993) reviewed histopathologic specimens from 11 patients, all children in whom the biopsy had been performed to exclude a malignant lesion. In no instance was the diagnosis of FOP considered before the biopsy. In 6 children the findings were misinterpreted as indicating fibromatosis or sarcoma, at an early stage before the roentgenographic appearance of ossification. Immunohistochemical studies of sections of the earliest lesion demonstrated S-100 antigen positivity before the histologic appearance of differentiated osteochondral tissue. The biopsies in all 11 patients showed endochondral osteogenesis that was normal except for the ectopic site. They suggested that biopsy is not required to make the diagnosis because of the consistency of the findings in the great toe, and indeed should be avoided since biopsy uniformly exacerbates the condition. Lin et al. (2006) stressed the importance of examining the feet of children with a history of heterotopic bone formation to arrive at the correct diagnosis and avoid the risk of further injury.
Kitterman et al. (2005) surveyed the 269 patient-members of the International FOP Association, comprising more than 90% of all known FOP patients worldwide, and received 138 responses to the questionnaire (51% response rate) from 25 countries. Incorrect diagnoses were given initially to 87% of individuals with FOP; the most common incorrect diagnosis was cancer. The mean period from onset of symptoms to correct diagnosis was 4.1 years, and the median number of physicians consulted prior to correct diagnosis was 6. Unnecessary invasive procedures (biopsies) were performed in 67% of patients, and 68% received inappropriate therapies. Almost half of the responders (49%) reported permanent loss of mobility resulting from invasive medical interventions that caused posttraumatic ossification. Kitterman et al. (2005) also reviewed 184 English-language textbooks in relevant specialties and found that only 8% contained adequate descriptions of FOP, including the caution that trauma can accelerate the process of heterotopic ossification. The authors concluded that iatrogenic harm resulting from diagnostic failures for this rare disorder is common worldwide and has shaped the natural history of the disease for most affected individuals.
Molecular GeneticsShore et al. (2006) conducted a genomewide linkage analysis using a subset of 5 families with the most stringent and unambiguous features of FOP, congenital malformation of the great toes and progressive heterotopic ossification in characteristic anatomic patterns, in all affected family members. This approach identified linkage of FOP to chromosome 2q23-q24, a genetic interval including the ACVR1 gene. Sequence analysis of all ACVR1 protein-coding exons and splice junctions identified a heterozygous mutation (R206H; 102576.0001) in all examined familial individuals, including all 5 families used for linkage analysis, and in 32 of 32 sporadic FOP patients with unambiguous clinical features. The examined individuals with the R206H mutation included an individual who was previously reported by Semonin et al. (2001) with an unverifiable mutation in the NOG gene (602991); see Xu et al. (2002, 2000).
In a 3-year-old Taiwanese girl with dysplasia of the first metatarsal bones and progressive heterotopic ossificans of the right thigh due to routine childhood immunizations and several inappropriate surgical interventions, Lin et al. (2006) identified a de novo R206H mutation in the ACVR1 gene.
Nakajima et al. (2007) identified the R206H mutation in 3 unrelated sporadic Japanese patients with FOP, indicating that this mutation is common and recurrent in the global population.
In a 62-year-old Japanese man with slowly progressive FOP, Furuya et al. (2008) identified heterozygosity for a de novo missense mutation (G356D; 102576.0002) in the ACVR1 gene. The mutation was not found in his unaffected sibs or in 150 controls. The authors suggested that the patient's longevity and slow progression of respiratory difficulties might be related to the position of the G356D mutation in the protein kinase domain rather than in the functionally important glycine/serine-rich domain where the R206H mutation is located.
Bocciardi et al. (2009) identified the R206H mutation in 15 of 17 unrelated Italian patients with FOP. In 2 unrelated Italian patients, they identified heterozygosity for a different mutation (R258S; 102576.0003). The authors noted that there was extreme variability among the 17 patients in the severity of the disease; in addition, of the 2 patients with the R258S mutation, 1 did not have the great toe malformation, and the other had it to a 'rather mild' degree.
Clinical Variability
Kaplan et al. (2009) studied 112 FOP patients, of whom 104 were sporadic cases and 8 were familial cases. Classic FOP was found in 82 sporadic cases and 7 familial cases, whereas 20 sporadic cases and 1 familial case had atypical FOP. The atypical patients formed 2 classes: so-called 'FOP-plus,' in which patients had the classic defining features of FOP (progressive heterotopic ossification and great toe malformations) plus 1 or more atypical features; and 'FOP variants,' in which there were major variations in 1 or both of the 2 classic defining features of FOP. The recurrent ACVR1 mutation R206H (102576.0001) was found in all of the patients with classic FOP and most of those with FOP-plus, whereas the G356D mutation (102576.0002) or novel ACVR1 mutations were identified in patients with FOP variants and in 2 cases of FOP-plus (see, e.g., 102576.0004-102576.0007). Kaplan et al. (2009) noted that all mutations in ACVR1 associated with FOP in any form were located in or adjacent to the GS regulatory region or active site of the kinase, and all were predicted by protein structure homology modeling to activate the ACVR1 protein and enhance receptor signaling.
In a 20-year-old woman with FOP, who had a later onset and relatively mild course of disease, Petrie et al. (2009) identified heterozygosity for an R202I mutation in the ACVR1 gene (102576.0008). In a 52-year-old woman with FOP, first reported by Smith et al. (1976) and later reviewed by Connor and Evans (1982), who was born with severe reduction deformities of all digits, Petrie et al. (2009) identified a heterozygous G328E mutation (102576.0008).
In a female patient with variant FOP, who had normal great toes and late-onset heterotopic ossification and was misdiagnosed with ankylosing spondylitis for several years, Barnett et al. (2011) identified heterozygosity for the R202I mutation in the ACVR1 gene.
In a 45-year-old woman with a late-onset, mild form of FOP, Gregson et al. (2011) identified heterozygosity for a missense mutation in the ACVR1 gene (L196P; 102576.0009). The patient, who had normal toes and bilateral mild camptodactyly of the fifth fingers, was 21 years old when she developed heterotopic ossification following a car accident. Asymptomatic early ossification of cervical spine facet joints was noted at age 42, and she also experienced recurrent episodes of inflammation without subsequent ossification. Gregson et al. (2011) stated that this patient had the most benign clinical course of any reported FOP case.
Nakahara et al. (2014) reported a 22-year-old Japanese man who exhibited delayed onset and a slower, milder course of FOP who was heterozygous for the L196P mutation in ACVR1. He was well until he fell from a height of 1 to 2 meters at 17 years of age and subsequently developed heterotopic ossification in the lumbar area. At 19 years of age, he noticed decreased range of motion in his right shoulder without any known injury; MRI showed a T2-weighted high-intensity lesion in the right scapular region. Examination at age 22 revealed limited range of motion of cervical and lumbar spine and at the hip joints. He had camptodactyly of the left fifth digit, short toes, and absence of the distal interphalangeal joint of the fourth and fifth toes bilaterally. X-rays showed mature heterotopic calcification bilaterally in the lumbar paraspinal muscles, mild osteosclerotic lesions bilaterally in the inner cortex of the proximal tibia, and slight enlargement of the C6 spinous process with narrowing of the C6-7 intervertebral joint.
HistoryAssociation between FOP and NOG
Although there have been reports indicating that mutations in the NOG gene (602991) cause FOP, numerous studies have refuted this association.
Because mutations in the NOG gene, located on chromosome 17, had been identified in proximal symphalangism (185800), which has some phenotypic similarities to the involvement of the digits in FOP, Lucotte et al. (1999) sequenced the NOG coding region in the FOP patient with normal parents who had been described by Daltroff et al. (1992) and found heterozygosity for a 42-bp deletion. Lucotte et al. (2000) subsequently performed linkage analysis in seven 2-generation families originating from the UK, France (3 families), Spain, Tunisia, and Syria and found linkage to chromosome 17q21-q22. However, in the patient studied by Lucotte et al. (1999) and in 26 other patients with sporadic FOP and 12 affected members of 4 families, Xu et al. (2000) could find no mutations in the NOG gene. Linkage analysis in 3 of 4 classically affected multigenerational FOP families excluded linkage of FOP to the NOG locus; in the remaining family, linkage to chromosome 17 could not be excluded. Xu et al. (2000) noted that the protein-coding region of the single-exon NOG gene is extremely GC-rich (67%), which suggests that the gene may be highly methylated and/or susceptible to secondary structure formation, conditions that interfere with the fidelity of PCR amplification and could plausibly explain the previously reported and subsequently unverifiable NOG deletion in the patient with FOP.
Lucotte et al. (2000) ascertained 38 French patients with FOP via questionnaires sent to general practitioners; 20 of those patients were analyzed in more detail using medical records and patient questionnaires. Only 2 probands representing possible familial cases and their family members were examined by the authors. The first proband examined was a 21-year-old woman who had skeletal malformation of the toes at birth and sternocleidomastoid ossification noted at 8 years of age. Her father and paternal great uncle had hallux valgus; however, radiologic examination of her father revealed no other bony abnormalities, suggesting that this was not a familial case. The other proband who was examined was a 47-year-old man with a 'limited' form of the disease, who was the only patient to have married; he had no skeletal malformation noted at birth, developed ossification of the left shoulder at 35 years of age, and had maxillary involvement. Both patients had biopsies but the results were not reported.
In 4 Spanish patients with FOP, Semonin et al. (2001) reported heterozygosity for 3 different mutations in the NOG gene. Xu et al. (2002) stated that these reported mutations in the NOG gene are PCR errors as described in their previous study (Xu et al., 2000). Warman (2002) suggested that the divergent results might arise from methodologic issues including possible phenotype error and/or the use of a nested PCR approach, which increases the likelihood of PCR-induced artifacts; he proposed that photographs and radiographs of the patients with FOP and NOG mutations be published and that DNA samples from patients with putative disease-causing FOP mutations be shared with other laboratories for independent confirmation using a different methodology.
In an Editor's Note commenting on the controversy regarding the involvement of the NOG gene in FOP, Carey (2002) stated that more clinical information on the patients studied by Semonin et al. (2001), including photographs and radiographs, would be welcome and would be published in AJMG; Carey (2002) also urged sharing of DNA samples for independent testing, as well as documentation of the functional studies mentioned by Fontaine et al. (2002) in their reply to Xu et al. (2002) and Warman (2002).
Using the disputed DNA sequencing techniques previously described by Semonin et al. (2001) involving a nested approach prone to PCR-induced artifacts (Xu et al., 2000; Warman, 2002), Lucotte et al. (2007) analyzed the NOG gene in 45 unrelated patients diagnosed with FOP and reported identification of 6 additional patients with a mutation in NOG. They also identified heterozygosity for the R206H mutation in the ACRV1 gene in 23 patients, 1 of whom had previously been reported to have a 42-bp deletion in the NOG gene (Lucotte et al., 1999) and another who had been reported to carry a 'rare polymorphism' in NOG (Fontaine et al., 2005).
Regarding the hypothesis by Lucotte et al. (2007) that the polyGly stretch of the NOG gene in which they identified mutations is a myristoylation site, Seemann and Mundlos (2008) stated that NOG is not an intracellular protein and is not processed at the polyGly stretch, and therefore is not a substrate for myristoylation; they concluded that experimental data in support of the myristoylation hypothesis were 'missing completely.' Noting that as of 2008, Lucotte had not answered the invitation from Carey (2002) to present complete phenotypic data on all of his patients with reported NOG mutations and to share DNA samples with other laboratories outside of his consortium so that independent testing could occur, Kaplan et al. (2008) concluded that there was no plausible evidence that mutations in the NOG gene cause FOP. In response, Semonin et al. (2008) reiterated their previous findings.
Mapping
Feldman et al. (2000) conducted a genomewide linkage analysis, using 4 affected families with a total of 14 informative meioses. Male-to-male transmission of the FOP phenotype excluded X-linked inheritance. Highly polymorphic microsatellite markers covering all human autosomes were amplified by use of PCR. Linkage of the FOP phenotype was found to the 4q27-q31 region (lod score = 3.10 at recombination fraction = 0.0). Crossover events localized the putative FOP gene within a 36-cM interval bordered proximally by D4S1625 and distally by D4S2417. Later studies by Shore et al. (2006) excluded linkage of these families to chromosome 4.
Animal ModelKan et al. (2004) generated mice overexpressing BMP4 under the control of the neuron-specific enolase promoter (ENO2; 131360) and observed the development of progressive postnatal heterotopic endochondral ossification, a phenotype that matches the anatomic, spatial, and temporal characteristics of human FOP. The phenotype was completely rescued in double-transgenic mice that also overexpressed noggin, confirming the role of BMP4 in the pathogenesis of the disease.
In murine experiments, Shimono et al. (2011) demonstrated that RARG (180190) agonists are potent inhibitors of intramuscular and subcutaneous heterotopic ossification. Transgenic mice expressing the Alk2 (102576) mutation Q207D, a constitutively active form of the receptor that is related to FOP-causing ALK2 R206H mutation (102576.0001), were treated with the RARG agonist CD1530. Heterotopic ossification was essentially prevented in the treated mutant mice, compared to massive heterotopic ossification that developed in control mice.