Wilson Disease
Summary
Clinical characteristics.
Wilson disease is a disorder of copper metabolism that can present with hepatic, neurologic, or psychiatric disturbances, or a combination of these, in individuals ranging from age three years to older than 50 years; symptoms vary among and within families.
- Liver disease includes recurrent jaundice, simple acute self-limited hepatitis-like illness, autoimmune-type hepatitis, fulminant hepatic failure, or chronic liver disease.
- Neurologic presentations include movement disorders (tremors, poor coordination, loss of fine-motor control, chorea, choreoathetosis) or rigid dystonia (mask-like facies, rigidity, gait disturbance, pseudobulbar involvement).
- Psychiatric disturbance includes depression, neurotic behaviors, disorganization of personality, and, occasionally, intellectual deterioration.
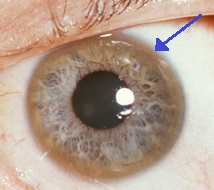
Kayser-Fleischer rings, frequently present, result from copper deposition in Descemet's membrane of the cornea and reflect a high degree of copper storage in the body.
Diagnosis/testing.
Wilson disease is suspected in a proband with varying combinations of hepatic, neurologic, and psychiatric findings. The diagnosis is established in most instances by a combination of biochemical findings (low serum copper and ceruloplasmin concentrations, and increased urinary copper excretion) and clinical findings (Kayser Fleischer corneal ring) or detection of biallelic ATP7B pathogenic variants on molecular genetic testing. Of note, when results from molecular genetic testing are not available to allow timely diagnosis, quantification of hepatic liver content (biopsy) may be required for diagnosis. In this instance, molecular genetic testing is strongly encouraged for confirmation of the diagnosis.
Management.
Treatment of manifestations: Treatment with copper chelating agents or zinc – initiated as soon as possible – can reduce hepatic, neurologic, and psychiatric findings in many symptomatic individuals. Treatment is life long. Copper chelating agents (D-penicillamine or trientine) increase urinary excretion of copper. High-dose oral zinc interferes with absorption of copper from the gastrointestinal tract and is most effective after initial decoppering with a chelating agent. Orthotopic liver transplantation is used for individuals who fail to respond to medical therapy or present with fulminant acute liver failure.
Prevention of primary manifestations: Treatment with copper chelating agents or zinc can prevent the development of hepatic, neurologic, and psychiatric findings in asymptomatic affected individuals.
Surveillance: At least twice annually: serum copper and ceruloplasmin, liver biochemistries, international normalized ratio, complete blood count, urinalysis, and physical examination including neurologic assessment. At least once annually: 24-hour urinary excretion of copper.
Agents/circumstances to avoid: Foods very high in copper (liver, brain, chocolate, mushrooms, shellfish, and nuts), especially at the beginning of treatment.
Evaluation of relatives at risk: If the pathogenic variants in an affected family member are known, molecular genetic testing of sibs of a proband allows early diagnosis and initiation of therapy before symptoms occur. If the pathogenic variants in an affected family member are not known, biochemical assessment of parameters of copper metabolism (serum copper, urinary copper, ceruloplasmin) and liver function tests as well as ultrasound imaging of the liver and slit lamp examination for the presence of Kayser-Fleischer rings can be conducted.
Pregnancy management: Treatment must be continued during pregnancy because of the risk for fulminant hepatic failure or irreversible neurologic deterioration. Because of possible adverse effects on the fetus from chelating agents, the dose should be kept as low as possible.
Genetic counseling.
Wilson disease is inherited in an autosomal recessive manner. At conception, each sib of an affected individual has a 25% chance of being affected, a 50% chance of being an asymptomatic carrier, and a 25% chance of being unaffected and not a carrier. Once the ATP7B pathogenic variants have been identified in an affected family member, carrier testing for at-risk relatives and prenatal testing and preimplantation genetic testing for pregnancies at increased risk for Wilson disease are possible.
Diagnosis
The diagnosis of Wilson disease cannot be made by a single test alone: a combination of tests is always required, as outlined in detail in the most current US guidelines, the American Association for the Study of Liver Diseases (AASLD) guidelines [Roberts & Schilsky 2008].
The diagnostic algorithm of the more recent European Association for Study of Liver (EASL) Clinical Practice Guidelines [European Association for Study of Liver 2012] is based on a diagnostic index ("Leipzig" score) proposed by an expert panel [Ferenci et al 2003]. This score includes clinical, biochemical, and molecular features, but has not been validated in large patient series.
Suggestive Findings
Wilson disease is suspected in individuals age three to 60 years (commonly 6-45 years) [Ferenci et al 2007] with varying combinations of hepatic, neurologic, psychiatric, and ocular findings.
- Liver disease includes recurrent jaundice, simple acute self-limited hepatitis-like illness, autoimmune-type hepatitis, fulminant hepatic failure, or chronic liver disease.
- Neurologic presentations include movement disorders (tremors, poor coordination, loss of fine-motor control, chorea, choreoathetosis) or rigid dystonia (mask-like facies, rigidity, gait disturbance, pseudobulbar involvement).
- Psychiatric disturbance includes depression, neurotic behaviors, disorganization of personality, and, occasionally, intellectual deterioration.
- Kayser-Fleisher rings, copper deposits in the periphery of the cornea, are observed in approximately 50%-60% of individuals with liver disease and about 90% of individuals with either neurologic findings or psychiatric disturbance. They are observed most reliably by slit lamp examination.
Establishing the Diagnosis
The diagnosis of Wilson disease is established in most instances by biochemical findings. If observed in combination with low ceruloplasmin levels, the presence of Kayser-Fleisher rings is almost pathognomonic. The diagnosis is confirmed by molecular genetic testing.
Note: If timing during diagnostic testing is an issue and if the results from molecular genetic testing may not be available immediately, quantification of hepatic iron content (on biopsy) may be required. In this instance, molecular genetic testing is also strongly encouraged for confirmation (see Table 2 in Ferenci et al [2003]).
Biochemical Findings
The biochemical diagnosis of Wilson disease in a symptomatic individual relies on a combination of the following findings:
- Low serum ceruloplasmin concentration
- In children, interpretation of test results requires age correction or age-specific reference ranges.Note: Healthy newborns have low serum ceruloplasmin concentrations. The concentrations increase during the first six months of life and peak by age two to three years at a concentration that may exceed the healthy adult reference range.
- In adults with Wilson disease, serum ceruloplasmin concentration is often below the normal range and typically very low.Note: A normal serum ceruloplasmin concentration is found in at least 5% of individuals with Wilson disease with neurologic symptoms and up to 40% of individuals with hepatic symptoms [Steindl et al 1997]. Serum ceruloplasmin concentration is, therefore, not a reliable screening test for Wilson disease.
- Serum concentration of copper and of non-ceruloplasmin-bound copper
- Most individuals with Wilson disease have a subnormal serum copper concentration that is proportional to the serum ceruloplasmin concentration.Note: Serum copper is low in healthy newborns. The concentrations increase during the first six months of life and by age two to three years peak at a concentration that may exceed the healthy adult reference range.
- The combination of low ceruloplasmin serum concentration and a normal or high serum copper concentration may suggest excess non-ceruloplasmin-bound copper in the serum. Such high non-ceruloplasmin-bound serum copper concentrations often present as a result of copper overload; however, it is not reliable for diagnosis because of its high dependency on the accuracy of both the serum ceruloplasmin concentration and the serum copper concentration.The serum concentration of non-ceruloplasmin-bound copper (in µg/L) is most reliably estimated by subtracting the amount of copper associated with ceruloplasmin, determined by the enzymatic assay (ceruloplasmin in mg/L x 3.15) from the total serum copper concentration. Normal serum concentration of non-ceruloplasmin-bound copper is approximately 50-100 µ/L. In individuals with Wilson disease, the serum concentration of non-ceruloplasmin-bound copper is usually higher than 200 µ/L.Note: Enzymatic methods for quantification of ceruloplasmin measure holoceruloplasmin (i.e., with copper incorporated) and are therefore preferred, particularly for calculation of the free copper concentration [Walshe 2003b, Macintyre et al 2004].
- High urinary copper. Measurement of copper in three 24-hour urine collections, free from contamination by external sources of copper, is advised. The testing laboratory should be consulted regarding its trace-element urine collection protocol prior to initiating urine specimen collection.
- Basal urinary copper excretion (without the use of chelating agent) is almost invariably elevated above 0.6 µmol/24 hours in the symptomatic individual.
- A provocative test of urinary copper excretion following oral administration of D-penicillamine has been validated only in pediatric cohorts, but has proven useful in many cases [Martins da Costa et al 1992], although levels in affected individuals can overlap with those of heterozygotes.
- Increased hepatic copper concentration. Hepatic copper concentration in Wilson disease is usually greater than 250 µg/g dry weight (normal: <55 µg/g dry weight [Nuttall et al 2003]); however, such levels may be seen in other chronic liver disorders and in cholestatic conditions as well.Note: (1) In later stages of Wilson disease, copper is distributed unevenly in the liver and measurement of hepatic copper concentration is less reliable. (2) Some individuals have only a moderately elevated hepatic copper concentration: 100 to 250 µg/g dry weight, which overlaps with values occasionally found in heterozygotes. Thus, hepatic copper concentration in this range does not exclude the diagnosis of Wilson disease.
Molecular Genetic Testing
The molecular diagnosis of Wilson disease relies on identification of biallelic pathogenic variants in ATP7B on molecular genetic testing (see Table 1).
Molecular genetic testing approaches can include single-gene testing, use of a multigene panel, and more comprehensive genomic testing.
Single-gene testing. Sequence analysis of ATP7B is performed first and followed by gene-targeted deletion/duplication analysis if only one or no pathogenic variant is found.
Targeted analysis for specific pathogenic variants can be performed first in individuals from specific populations:
- Populations of European origin. p.His1069Gln accounts for 35%-45% of Wilson disease-causing alleles in a mixed European population [Tanzi et al 1993] and a greater percent in Eastern Europe [Caca et al 2001]. The frequency of this pathogenic variant may be somewhat lower in probands with childhood onset and in probands presenting with liver disease.
- Asian populations. p.Arg778Leu is the only relatively common pathogenic variant, accounting for approximately 57% of Wilson disease-causing alleles in the Asian population younger than age 18 years [Thomas et al 1995].
- In Sardinia. A single pathogenic variant, a15-bp deletion in the 1-kb promoter region (c.-441_-427del15) is common [Loudianos et al 1999].
A multigene panel that includes ATP7B and other genes of interest (see Differential Diagnosis) may also be considered. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview; thus, clinicians need to determine which multigene panel is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. (3) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.
For an introduction to multigene panels click here. More detailed information for clinicians ordering genetic tests can be found here.
More comprehensive genomic testing (when available) including exome sequencing, mitochondrial sequencing, and genome sequencing may be considered if single-gene testing (and/or use of a multigene panel that includes ATP7B) fails to confirm a diagnosis in an individual with features of Wilson disease. Such testing may provide or suggest a diagnosis not previously considered (e.g., mutation of a different gene or genes that results in a similar clinical presentation). For an introduction to comprehensive genomic testing click here. More detailed information for clinicians ordering genomic testing can be found here.
Table 1.
Molecular Genetic Testing Used in Wilson Disease
| Gene 1 | Method | Proportion of Probands with Pathogenic Variants 2 Detectable by Method |
|---|---|---|
| ATP7B | Sequence analysis 3 | 98% 4 |
| Gene-targeted deletion/duplication analysis 5 | Rare 6 |
- 1.
See Table A. Genes and Databases for chromosome locus and protein.
- 2.
See Molecular Genetics for information on allelic variants detected in this gene.
- 3.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 4.
Stättermayer et al [2014]
- 5.
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications.
- 6.
Large deletions and duplications, encompassing one or more exons, are rare. Exon and multiexon deletions have been reported (see, e.g., Møller et al [2005], Incollu et al [2011], Møller et al [2011], Tatsumi et al [2011].
Clinical Characteristics
Clinical Description
Wilson disease can manifest as hepatic, neurologic, hematologic, or psychiatric disturbances, or a combination of these, in individuals ranging in age from three years to older than 60 years. Phenotypic expression varies even within families. The phenotypic spectrum has further expanded through molecular genetic testing, which has confirmed the diagnosis in individuals with atypical clinical and biochemical findings [Cox & Roberts 2006, Ala et al 2007, Bandmann et al 2015].
Table 2 outlines the typical clinical findings of Wilson disease. Of note, the "classic triad" of liver disease, movement disorder, and Kayser-Fleischer ring is uncommon.
Table 2.
Clinical Findings in Individuals with Wilson Disease by Presenting Finding
| Presenting Finding | % of Individuals | Typical Age of Presentation (Range) | Liver Disease | Neurologic Disease | Psychiatric Disturbance | Kayser-Fleischer Rings |
|---|---|---|---|---|---|---|
| Liver disease | ~40% | 6-45 (3-70) | + | +/– | +/– | ~50% |
| Neurologic disease | ~40% | Mid-teen to mid-adult (6-50) | –/mild | + | +/– | ~90% |
| Psychiatric disturbance | ~20% | Adolescent to young adult | –/mild | +/– | + | ~90% |
| Hemolytic anemia | Few % | Adolescent to young adult | + | – | – | + |
Bruha et al [2011], Weiss et al [2011], Hofer et al [2012], Weiss et al [2013b]
Liver disease. Wilson disease manifests as liver disease more commonly in children and younger adults, typically between ages six and 45 years; however, severe liver disease can be the initial finding in preschool-aged children [Wilson et al 2000] and in older adults. The clinical manifestations vary and can include the following findings:
- Recurrent jaundice, possibly caused by hemolysis
- Simple, acute, self-limited hepatitis-like illness with fatigue, anorexia, abdominal pain
- Autoimmune hepatitis, often manifest acutely with fatigue, malaise, arthropathy, and rashes. This form of liver disease responds well to chelation therapy even if cirrhosis is present (see Management).
- Fulminant hepatic failure with severe coagulopathy, encephalopathy, acute Coombs-negative intravascular hemolysis, and often rapidly progressive renal failure. Serum activity of aminotransferases is only moderately increased, and serum concentration of alkaline phosphatase is normal or extremely low. These individuals do not respond to chelation treatment and require urgent liver transplantation (see Management).
- Chronic liver disease with portal hypertension, hepatosplenomegaly, ascites, low serum albumin concentration, and coagulopathy
- Fatty liver of mild to moderate degree with abnormal liver function
- Hemolytic anemia, with either acute or chronic hemolysis, a reflection of a high serum concentration of non-ceruloplasmin-bound copper, which leads to destruction of erythrocytes. Liver disease is likely to be present in such individuals, as are Kayser-Fleischer rings. Recurrent hemolysis predisposes to cholelithiasis, even in children.
Neurologic disease. Neurologic involvement follows two general patterns: movement disorders or rigid dystonia.
- Movement disorders tend to occur earlier and include tremors, poor coordination, loss of fine-motor control, micrographia (abnormally small, cramped handwriting), chorea, and/or choreoathetosis.
- Spastic dystonia disorders manifest as mask-like facies, rigidity, and gait disturbance [Svetel et al 2001].
Pseudobulbar involvement such as dysarthria, drooling, and difficulty swallowing is more common in older individuals, but also occurs in children and adolescents.
In contrast to the neurologic findings in individuals with a frank neurologic presentation, the neurologic findings in individuals with a hepatic presentation may be subtle. Mood disturbance (mainly depression; occasionally poor impulse control), changes in school performance, and/or difficulty with fine motor skills (especially handwriting) or gross motor skills may be observed.
Psychiatric manifestations. The psychiatric manifestations are variable. Depression is common. Neurotic behavior includes phobias, compulsive behaviors, aggression, or antisocial behavior. Older individuals may have subtle psychopathology (e.g., progressive disorganization of personality with anxiety) and affective changes (e.g., labile mood and disinhibition). Intellectual deterioration may also occur with poor memory, difficulty in abstract thinking, and shortened attention span. Pure psychotic disorders are uncommon.
Kayser-Fleischer rings result from copper deposition in Descemet's membrane of the cornea, and reflect a high degree of copper storage in the body. They do not affect vision and are reduced or disappear with effective decoppering treatment (see Management).
Other findings
- Renal involvement. Low-molecular weight proteinuria, microscopic hematuria, and Fanconi syndrome
- Arthritis. Involvement of large joints from synovial copper accumulation
- Reduced bone mineral density with a prevalence of osteoporosis in approximately 10% of affected individuals
- Pancreatitis, cardiomyopathy, cardiac arrhythmias, rhabdomyolysis of skeletal muscle, and various endocrine disorders
- Sunflower cataracts observed occasionally on slit lamp examination
Hepatocellular carcinoma rarely develops in Wilson disease: the estimated incidence is below 1% [Devarbhavi et al 2012]. However, abdominal malignancies have been reported in treated individuals [Walshe et al 2003].
Fertility and pregnancy. Most individuals with Wilson disease are fertile. Successful pregnancies of women with Wilson disease who received treatment have been reported [Brewer et al 2000, Tarnacka et al 2000, Furman et al 2001]. Prior to diagnosis and treatment, affected women may experience infertility or recurrent miscarriage.
Genotype-Phenotype Correlations
Pathogenic variants that abolish ATP7B function tend to result in a more severe phenotype than some missense variants [Cox 1996, Deguti et al 2004, Liu et al 2004, Panagiotakaki et al 2004].
Several studies have found a mean age of onset of 20 to 22 years in individuals homozygous for the common p.His1069Gln pathogenic variant [Stapelbroek et al 2004], although earlier onset also occurs.
Marked differences between disease severity and clinical features in sibs suggest that the clinical outcome is influenced by modifying factors. It has been proposed that the clinical phenotype of Wilson disease is modified by pathogenic variants in other genes including MTHFR (encoding methylenetrahydrofolate reductase) [Gromadzka et al 2011], COMMD1 [Weiss et al 2006], ATOX1 [Simon et al 2008], and XIAP [Weiss et al 2010]. Although some minor associations have been reported, to date none of these genes is clinically relevant or has a significant diagnostic or predictive value.
Nomenclature
The neurologic form of Wilson disease has also been known as Westphal-Strumpell pseudosclerosis.
Prevalence
The prevalence of Wilson disease is estimated at one in 30,000 in most populations, with a corresponding carrier frequency in the general population of one in 90 [Bachmann et al 1991, Reilly et al 1993, Olivarez et al 2001].
Recent studies suggest a prevalence as high as one in 10,000 [Coffey et al 2013], especially in isolated populations such as Sardinia [Gialluisi et al 2013].
Differential Diagnosis
Other liver diseases presenting with abnormal liver biochemistries with or without hepatomegaly that need to be considered include the following:
- Chronic viral hepatitis
- Autoimmune hepatitis
- Non-alcoholic steatohepatitis (NASH)Note: Wilson disease must be specifically excluded in individuals thought to have NASH or the opportunity for life-saving treatment will be missed.
- Primary sclerosing cholangitis (OMIM 613806)
- Drug hepatotoxicity
- HFE-associated hereditary hemochromatosis
- Alpha-1-antitrypsin deficiency
- Alcoholic liver disease
- Primary biliary cirrhosis (OMIM 109720)
Other liver diseases presenting as fulminant hepatic failure that need to be considered are acute viral hepatitis of any etiology and severe drug toxicity.
Kayser-Fleischer rings are not specific for Wilson disease and may in extremely rare cases be seen in copper accumulation associated with cholestatic liver diseases or autoimmune hepatitis.
Subnormal serum concentration of ceruloplasmin is not per se specific for Wilson disease, as ceruloplasmin synthesis can be reduced with acute liver failure or decompensated cirrhosis of any etiology. Decreased serum concentrations of ceruloplasmin are observed in protein-losing enteropathy, nephrotic syndrome, and malnutrition, but also in some heterozygotes for Wilson disease.
Serum concentration of ceruloplasmin is physiologically low in neonates.
Almost complete absence of ceruloplasmin is found in hereditary aceruloplasminemia, which results in iron storage [Miyajima et al 1987, Yoshida et al 1995].
Elevated liver copper content greater than 250 µg/g dry weight may be seen in other chronic liver disorders as well. As copper is secreted exclusively via the bile, hepatic copper concentration cannot be used as a diagnostic finding in persons with chronic cholestasis or impaired biliary excretion.
Familial/environmental copper storage diseases not related to Wilson disease have been identified but are rare; the most common of these is Indian childhood cirrhosis.
Other neurologic disorders that need to be considered:
- Benign familial or essential tremors
- Parkinson disease and its differential diagnoses, including:
- Huntington disease
- Dentatorubro-pallidoluysian atrophy (DRPLA)
- Juvenile Parkinson disease, including Parkin type of early onset parkinsonism
- Inherited forms of dystonia (see Dystonia Overview), including:
- DYT1 early-onset primary dystonia
- Dopa-responsive dystonia (DRD)
- Neurodegenerative diseases
- Drug effects or toxicity
- Hyperthyroidism
- Central nervous system neoplasia
- Hereditary ataxias
- Niemann-Pick disease type C (associated with liver disease)
Management
Evaluations Following Initial Diagnosis
To establish the extent of disease and needs in an individual diagnosed with Wilson disease, the following evaluations are recommended:
- Evaluation of severity of the liver disease by liver biopsy or by biochemical testing and imaging of the liver
- Upper GI endoscopy to exclude or confirm esophageal varices
- Detailed clinical neurologic assessment. A validated neurologic rating scale is available [Członkowska et al 2007].
- Brain MRI to assess for structural alteration
- Assessment of kidney function
- Consultation with a clinical geneticist and/or genetic counselor
Treatment of Manifestations
The goal of therapy is to institute treatment with chelating agents as soon as possible in individuals with symptomatic Wilson disease. See extensive review by the American Association for the Study of Liver Diseases [Roberts & Schilsky 2008] (full text) and EASL Clinical Practice Guidelines: Wilson's disease [European Association for Study of Liver 2012] (full text).
- Treatment is life long, including during pregnancy.
- If one treatment modality is discontinued, an alternative modality must be substituted.
- Discontinuation of all treatment leads to hepatic and neurologic decompensation, which is usually refractory to further medical intervention.
Copper chelating agents that increase urinary excretion of copper are the first-line treatment for persons with symptomatic Wilson disease. Note: Routine institution of chelation therapy before age three years has not been adequately assessed and may have adverse effects on growth.
- D-penicillamine (chelator). Used since the 1950s as first-line therapy for Wilson disease [Durand et al 2001, Walshe 2003a], D-penicillamine is given as tablets by mouth two or three times daily. Pyridoxine must be given along with D-penicillamine. Twenty-four-hour urine copper excretion is used to confirm chelation and increased excretion of copper. Urinary copper values should be five to ten times normal; if the values are lower, non-compliance may be an issue, or body copper stores may have been adequately depleted.
- Complete blood count and urinalysis must be monitored regularly during D-penicillamine therapy. Serious side effects can occur in up to 30% of individuals, and include: severe thrombocytopenia, leukopenia, aplastic anemia, proteinuria, nephrotic syndrome, polyserositis, Goodpasture syndrome, and severe skin reactions. An early allergic reaction with fever, rash, and proteinuria may occur. Evidence of any such side effects may require discontinuation of D-penicillamine and substitution of an alternate treatment. If such alternate therapies are unavailable, D-penicillamine-induced adverse events may be manageable by co-administration of steroids.
- D-penicillamine inhibits collagen cross-linking and has some immunosuppressant properties. After decades of treatment, individuals may have abnormal skin and connective tissue collagen, and possible chronic depletion of copper and (possibly) other trace metals.
- D-penicillamine should NOT be used simultaneously with zinc, pending adequate clinical assessment of this treatment strategy.
- Trientine (chelator), also known as triethylene tetramine dihydrochloride (2,2,2-tetramine) or trien, is the usual second-line treatment for individuals who cannot tolerate D-penicillamine. It is gaining acceptance as a first-line drug because of good efficiency and better tolerance than D-penicillamine; however, it is still not generally available in all countries.
- Complete blood count and urinalysis must be monitored regularly in all individuals on trientine.
- Rare side effects include gastritis with nausea and, in cases of overtreatment, iron deficiency anemia.
- Trientine should NOT be used simultaneously with zinc pending adequate assessment of this combination. Current reports suggest that the combination of trientine and zinc, temporally dispersed throughout the day such that each drug is administered 5-6 hours apart from the other, may be effective in severely decompensated hepatic Wilson disease [Santos Silva et al 1996, Askari et al 2003].
Zinc (metallothionein inducer). High-dose oral zinc interferes with absorption of copper from the gastrointestinal tract presumably by inducing enterocyte metallothionein, which preferentially binds copper from the intestinal contents and is lost in the feces as enterocytes are shed in normal turnover. Zinc therapy is most effective after initial decoppering with a chelating agent [Brewer 2001, Brewer et al 2001]. In selected cases, it can be used as an initial treatment [Milanino et al 1992, Linn et al 2009]. Zinc is taken as tablets by mouth at least twice (usually 3x) daily, before meals. The dose is based on the elemental zinc in the tablet. Twenty-four-hour urine copper excretion is used to monitor total body copper stores, which should decrease. Increase of urinary copper excretion under zinc therapy may indicate insufficient treatment efficacy [Weiss et al 2011]. The computed estimate of non-ceruloplasmin-bound copper may be used to titrate the zinc dose. Serum or urinary zinc concentration can be measured to monitor compliance in individuals taking zinc.
Note: (1) Gastritis, a common side effect, can be reduced with the use of zinc acetate or zinc gluconate; (2) zinc should NOT be used simultaneously with any chelator, pending further clinical investigation.
Antioxidants. Serum and hepatic vitamin E concentrations are reported to be low in individuals with Wilson disease [Sokol et al 1994, Ogihara et al 1995], likely because of excessive consumption to counteract free radicals produced by excess copper. Antioxidants such as vitamin E may be used along with a chelator or zinc in protecting tissues from damage.
Restriction of foods very high in copper (liver, brain, chocolate, mushrooms, shellfish, and nuts) is likely prudent, especially at the beginning of treatment. It is recommended that individuals with special dietary needs (e.g., vegetarians) consult with a trained dietitian.
Orthotopic liver transplantation (OLT) is reserved for individuals who fail to respond to medical therapy or cannot tolerate it because of serious adverse side effects [Schilsky et al 1994, Emre et al 2001, Sutcliffe et al 2003]. It remains controversial whether orthotopic liver transplantation should be a primary treatment for individuals with Wilson disease who have severe neurologic disease [Medici et al 2005, Weiss et al 2013a].
Prevention of Primary Manifestations
Medical therapy is recommended for asymptomatic patients to prevent development of symptoms (see Treatment of Manifestations).
Prevention of Secondary Complications
Monitoring of patients under therapy should include routine assessments of treatment efficacy by biochemical testing and clinical evaluation:
- Insufficient therapy, underdosage, or malcompliance could lead to reaccumulation of copper and development of new symptoms.
- Adverse events related to medical treatment (especially under D-penicillamine treatment) should be evaluated.
- Excessive long-term treatment could result in copper deficiency, leading to immobilization of iron (as observed in aceruloplasminemia) and to neurologic symptoms of copper deficiency [Horvath et al 2010, da Silva-Júnior et al 2011].
Surveillance
According to current guidelines (AASLD [Roberts & Schilsky 2008] and EASL Clinical Practice Guidelines [European Association for Study of Liver 2012]), routine monitoring should include the following examinations:
- At least twice annually: serum copper and ceruloplasmin, liver biochemistries, international normalized ratio, complete blood count, urinalysis, and physical examination including neurologic assessmentNote: Patients receiving chelation therapy require a complete blood count and urinalysis regularly, no matter how long they have been on treatment
- At least once annually: 24-hour urinary excretion of copperNote: Measurements are recommended more frequently if there are questions on compliance or if dosage of medications is adjusted.
Agents/Circumstances to Avoid
Foods very high in copper (liver, brain, chocolate, mushrooms, shellfish, and nuts) should be avoided, especially at the beginning of treatment.
Evaluation of Relatives at Risk
The goal is to identify those sibs of a proband who have Wilson disease preferably before symptoms occur so that the therapies described under Treatment of Manifestations can be initiated as soon as possible. Evaluations can include the following:
- Molecular genetic testing if both ATP7B pathogenic variants in the proband are known
- If the pathogenic variants in an affected family member are not known, biochemical assessment of parameters of copper metabolism (serum copper, urinary copper, ceruloplasmin) and liver function tests as well as ultrasound imaging of the liver and slit lamp examination for the presence of Kayser-Fleischer ringsNote: Because presymptomatic individuals generally have a low serum concentration of ceruloplasmin and mildly increased basal 24-hour urinary copper excretion, biochemical testing can be used; however, sometimes asymptomatic affected individuals cannot be distinguished from heterozygotes.
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Pregnancy Management
Treatment must be continued during pregnancy because of the risk of fulminant hepatic failure.
- D-penicillamine has been used in many pregnancies with no adverse outcomes; however, congenital connective tissue disorders encompassing inguinal hernias and skin laxity have been reported in some exposed infants. Such adverse outcomes may depend on dose, which should be kept as low as possible. The dose of D-penicillamine should be maintained at the lowest effective dose with the plan to reduce by approximately 30% in the third trimester if the mother has been well chelated prior to pregnancy. A possible over-chelated (copper deficiency) status prior to pregnancy or genetic characteristics of the mother can contribute to fetal abnormalities [Pinter et al 2004].
- Trientine has been used successfully during pregnancy, but the total number of reported cases is small. Reduction of the dose to the lowest effective dose is recommended using a comparable approach to that for D-penicillamine.
- Zinc has been used effectively during pregnancy.
See MotherToBaby for further information on medication use during pregnancy.
Therapies Under Investigation
Ammonium tetrathiomolybdate (chelator) interferes with copper absorption from the intestine and binds plasma copper with high affinity. It may be useful for treatment of severe neurologic Wilson disease because, unlike D-penicillamine, it appears not to be associated with early neurologic deterioration [Brewer et al 2003]. However, the ammonium salt has not proven suitable for oral formulations.
Choline tetrathiomolybdate (chelator) is a more stable salt formulation of tetrathiomolybdate and is currently under investigation for Wilson disease [Weiss et al 2015].
Curcumin. Experimental in vitro studies suggest partially restored protein expression of some ATP7B mutants by curcumin [van den Berghe et al 2009]. This could enable novel treatment strategies in Wilson disease by directly enhancing the protein expression of mutated ATP7B with residual copper export activity. Furthermore, curcumin is an ideal antioxidant and an effective scavenger of reactive oxygen species and can act as a copper chelating agent. However, clinical data in patients with Wilson disease are not yet available.
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for access to information on clinical studies for a wide range of diseases and conditions.