Merrf
Summary
Clinical characteristics.
MERRF (myoclonic epilepsy with ragged red fibers) is a multisystem disorder characterized by myoclonus (often the first symptom) followed by generalized epilepsy, ataxia, weakness, exercise intolerance, and dementia. Onset can occur from childhood to adulthood, occurring after normal early development. Common findings are ptosis, hearing loss, short stature, optic atrophy, cardiomyopathy, cardiac dysrhythmias such as Wolff-Parkinson-White syndrome, and peripheral neuropathy. Pigmentary retinopathy, optic neuropathy, diabetes mellitus, and lipomatosis have been observed.
Diagnosis/testing.
A clinical diagnosis of MERRF can be established in a proband with the following four "canonic" features: myoclonus, generalized epilepsy, ataxia, and ragged red fibers (RRF) in the muscle biopsy. A molecular diagnosis is established in a proband with suggestive findings and a pathogenic variant in one of the genes associated with MERRF. The m.8344A>G pathogenic variant in the mitochondrial gene MT-TK is present in more than 80% of affected individuals with typical findings. Pathogenic variants in MT-TF, MT-TH, MT-TI, MT-TL1, MT-TP, MT-TS1, and MT-TS2 have also been described in a subset of individuals with MERRF.
Management.
Treatment of manifestations: Ubiquinol, carnitine, alpha lipoic acid, vitamin E, vitamin B complex, and creatine may be of benefit to some individuals; traditional anticonvulsant therapy per neurologist for seizures; levetiracetam or clonazepam for myoclonus; physical therapy to improve any impaired motor function; aerobic exercise; standard pharmacologic therapy for cardiac symptoms; hearing aids or cochlear implants for hearing loss; diabetes mellitus treatment per endocrinologist.
Prevention of primary manifestations: Coenzyme Q10 (50-200 mg 2-3x/day), L-carnitine (1000 mg 2-3x/day), alpha lipoic acid, vitamin E, vitamin B supplements, and creatine, often used to improve mitochondrial function, have been of modest benefit in some individuals. Doses for children should be adjusted appropriately.
Surveillance: Routine evaluations every six to 12 months initially; annual neurologic, ophthalmologic, cardiology (electrocardiogram and echocardiogram), and endocrinologic evaluations (fasting blood sugar and TSH); audiology evaluations every two to three years.
Agents/circumstances to avoid: Mitochondrial toxins (e.g., aminoglycoside antibiotics, linezolid, cigarettes, alcohol); valproic acid should be avoided in the treatment of seizures.
Pregnancy management: During pregnancy, affected or at-risk women should be monitored for diabetes mellitus and respiratory insufficiency, which may require therapeutic interventions.
Genetic counseling.
MERRF is caused by pathogenic variants in mtDNA and is transmitted by maternal inheritance. The father of a proband is not at risk of having the mtDNA pathogenic variant. The mother of a proband usually has the mtDNA pathogenic variant and may or may not have symptoms. A male with a mtDNA pathogenic variant cannot transmit the pathogenic variant to any of his offspring. A female with a mtDNA pathogenic variant (whether symptomatic or asymptomatic) transmits the pathogenic variant to all of her offspring. Prenatal testing and preimplantation genetic testing for MERRF are possible if a mtDNA pathogenic variant has been detected in the mother. However, because the mutational load in embryonic and fetal tissues sampled (i.e., amniocytes and chorionic villi) may not correspond to that of all fetal tissues and because the mutational load in tissues sampled prenatally may shift in utero or after birth as a result of random mitotic segregation, prediction of the phenotype from prenatal studies is not possible.
Diagnosis
Clinical diagnostic criteria for MERRF (myoclonic epilepsy with ragged red fibers) have been published [Finsterer et al 2018] (see Establishing the Diagnosis).
Suggestive Findings
MERRF (myoclonic epilepsy with ragged red fibers) should be suspected in individuals with the following features.
Clinical features
- Myoclonus
- Generalized epilepsy
- Ataxia
- Myopathy
- Exercise intolerance
- Dementia
- Ptosis
- Sensorineural hearing loss
- Short stature
- Optic atrophy
- Peripheral neuropathy
Less common clinical signs (seen in <50% of affected individuals) include the following:
- Cardiomyopathy
- Pigmentary retinopathy
- Pyramidal signs
- Ophthalmoparesis
- Multiple lipomas
Laboratory features
- Lactic acidosis both in blood and in the CSF. In individuals with MERRF, the concentrations of lactate and pyruvate are commonly elevated at rest and increase excessively after moderate activity.Note: Other situations (unrelated to the diagnosis of MERRF or other mitochondrial diseases) in which lactate and pyruvate can be elevated are acute neurologic events such as seizure or stroke.
- Elevated CSF protein concentration. The concentration of CSF protein may be increased but rarely surpasses 100 mg/dL.
- Respiratory chain studies. Biochemical analysis of respiratory chain enzymes in muscle extracts usually shows decreased activity of respiratory chain complexes containing mtDNA-encoded subunits, especially COX deficiency. However, biochemical studies may also be normal.
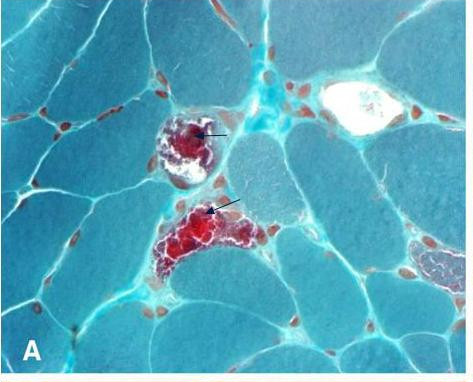
Histopathologic features on muscle biopsy. Ragged red fibers (RRF) are seen with the modified Gomori trichrome stain and hyperactive fibers with the succinate dehydrogenase stain. Both RRF and some non-RRF fail to stain with the histochemical reaction for cytochrome c oxidase. Occasionally, RRF may not be observed [Mancuso et al 2007].
Electrophysiologic features
- Electroencephalogram usually shows generalized spike and wave discharges with background slowing, but focal epileptiform discharges may also be seen.
- Electrocardiogram often shows pre-excitation; heart block has not been described.
- Electromyogram and nerve conduction velocity studies are consistent with a myopathy, but neuropathy may coexist.
Brain imaging. Brain MRI often shows brain atrophy and basal ganglia lesions. Bilateral putaminal necrosis and atrophy of the brain stem and cerebellum have been reported [Orcesi et al 2006, Ito et al 2008].
Establishing the Diagnosis
The clinical diagnosis of MERRF (myoclonic epilepsy with ragged red fibers) can be established in a proband based on clinical diagnostic criteria [Finsterer et al 2018] or the molecular diagnosis can be established in a proband with suggestive findings and a pathogenic variant in one of the genes listed in Table 1, identified by molecular genetic testing.
Clinical diagnosis. The clinical diagnosis is based on the following four "canonic" features:
- Myoclonus
- Generalized epilepsy
- Ataxia
- Ragged red fibers (RRF) in the muscle biopsy
Molecular diagnosis. The diagnosis of MERRF is established in a proband with suggestive findings and a pathogenic variant in one of the genes listed in Table 1.
Note: Pathogenic variants can usually be detected in mtDNA from leukocytes in individuals with typical MERRF; however, the occurrence of "heteroplasmy" in disorders of mtDNA can result in varying tissue distribution of mutated mtDNA. Hence, the pathogenic variant may be undetectable in mtDNA from leukocytes and may be detected only in other tissues, such as buccal mucosa, cultured skin fibroblasts, hair follicles, urinary sediment, or (most reliably) skeletal muscle.
Molecular genetic testing approaches can include a combination of gene-targeted testing (single-gene testing, concurrent or serial single-gene testing, multigene panel) and comprehensive genomic testing (exome sequencing, exome array, genome sequencing) depending on the phenotype.
Gene-targeted testing requires that the clinician determine which gene(s) are likely involved, whereas genomic testing does not. Individuals with the distinctive findings described in Suggestive Findings are likely to be diagnosed using gene-targeted testing (see Option 1), whereas those with a phenotype indistinguishable from many other inherited disorders with seizures and weakness are more likely to be diagnosed using genomic testing (see Option 2).
Option 1
Serial single-gene testing can be considered if (1) mutation of a particular gene accounts for a large proportion of the condition or (2) clinical findings, laboratory findings, ancestry, or other factors indicate that mutation of a particular gene is most likely.
Targeted analysis. Typically, blood leukocyte DNA is initially screened for pathogenic variants in MT-TK using targeted analysis for the m.8344A>G pathogenic variant, which is present in more than 80% of individuals with typical clinical findings. Note: If no pathogenic variant is found, consider targeted analysis for this pathogenic variant on DNA from buccal mucosa, muscle, or urine sediment.
Entire mitochondrial genome sequencing that includes the genes in Table 1 and other mtDNA genes of interest (Differential Diagnosis) is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype.
A multigene panel that includes the genes in Table 1 and other genes of interest (see Differential Diagnosis) may also be considered. Note: (1) The genes included and the sensitivity of multigene panels vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview; thus, clinicians need to determine which multigene panel is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.
For an introduction to multigene panels click here. More detailed information for clinicians ordering genetic tests can be found here.
Option 2
When the phenotype is indistinguishable from many other inherited disorders characterized by seizures and weakness, comprehensive genomic testing, which does not require the clinician to determine which gene is likely involved, is the best option. Exome sequencing is most commonly used; genome sequencing is also possible. Many laboratories require that the clinician specify if the mitochondrial genome should be included as part of the comprehensive genomic testing.
For an introduction to comprehensive genomic testing click here. More detailed information for clinicians ordering genomic testing can be found here.
Table 1.
Molecular Genetic Testing Used in MERRF
| Gene 1, 2 | % of MERRF Attributed to Pathogenic Variants in Gene | Proportion of Pathogenic Variants 3 Detectable by Sequence Analysis 4 |
|---|---|---|
| MT-TK | >90% 5 | 100% |
| MT-TF | <5% | 100% |
| MT-TH | ||
| MT-TI | ||
| MT-TL1 | ||
| MT-TP | ||
| MT-TS1 | ||
| MT-TS2 | ||
| Unknown 6 | NA |
- 1.
Genes are listed from most frequent to least frequent genetic cause of MERRF.
- 2.
See Table A. Genes and Databases for chromosome locus and protein.
- 3.
See Molecular Genetics for information on pathogenic allelic variants detected.
- 4.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, partial-, whole-, or multigene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 5.
Four MT-TK pathogenic variants (m.8344A>G, m.8356T>C, m.8363G>A, and m.8361G>A) account for approximately 90% of pathogenic variants in individuals with MERRF.
- 6.
One child with MERRF was found to have two mtDNA deletions in a buccal swab, suggesting an autosomal disorder with multiple mtDNA deletions; however, the causative nuclear gene was not identified [Yorns et al 2012].
Clinical Characteristics
Clinical Description
MERRF (myoclonic epilepsy with ragged red fibers) is a multisystem disorder characterized by myoclonus, which is often the first symptom, followed by generalized epilepsy, ataxia, weakness, exercise intolerance, and dementia. Onset can occur from childhood to adulthood, after normal early development. Table 2 lists the most frequent signs and symptoms reported [Hirano & DiMauro 1996, Mancuso et al 2013].
Table 2.
MERRF: Frequency of Select Features
| Feature | % of 62 Persons w/Feature 1 | % of 34 Persons w/Feature 2 | % of 321 Persons w/Feature 3 |
|---|---|---|---|
| Myoclonus | 100% | 24% | 61% |
| Epilepsy | 100% | 35% | 43% |
| Normal early development | 100% | ||
| Ragged red fibers | 92% | 96% | |
| Hearing loss | 91% | 35% | 39% |
| Lactic acidosis | 83% | 65% | |
| Family history of MERRF | 81% | ||
| Exercise intolerance | 80% | 44% | |
| Dementia | 75% | 25% | |
| Neuropathy | 63% | 15% | 24% |
| Short stature | 57% | ||
| Impaired sensation | 50% | ||
| Optic atrophy | 39% | ||
| Cardiomyopathy | 33% | 12% | |
| Arrhythmia | 22% | 18% | |
| Pigmentary retinopathy | 15% | ||
| Pyramidal signs | 13% | ||
| Ophthalmoparesis | 11% | 6% | 6% |
| Lipomatosis | 3% | 32% | 8% |
| Diabetes mellitus | 12% | 3% |
- 1.
Hirano & DiMauro [1996]
- 2.
Mancuso et al [2013]
- 3.
Reviewed from the literature by Mancuso et al [2013]
Neurologic manifestations
- Myopathy. The most common features are exercise intolerance, muscle weakness, and elevated blood creatine kinase level.
- Epilepsy. Generalized myoclonic seizures are the most frequent seizure type in individuals with MERRF. Other reported types of seizures include focal myoclonic, focal atonic, focal clonic, generalized tonic-clonic, generalized atonic, generalized myoclonic-atonic, typical absences, myoclonic absences, or tonic-clonic seizures of unknown onset [Finsterer & Zarrouk-Mahjoubb 2017].
- Migrainous headaches. Migraines are common in individuals with MERRF and can be present at the onset of symptoms. Vollono et al [2018] reported seven individuals with MERRF and migraines and found that migraines can present with or without aura. The frequency of migraine episodes in most individuals was every two months.
- Hearing impairment. Hearing loss is typically sensorineural and occurs in 35%-91% of individuals.
- Peripheral neuropathy. It has been reported that either sensory axonal neuropathy or sensory motor axonal neuropathy can be present.
- Early psychomotor development. Early development is typically normal in individuals with MERRF.
- Psychiatric illnesses. Although psychiatric illness is not a prominent feature in this disorder, several individuals with depressive mood disorders have been reported.
Cardiac involvement. Cardiomyopathy can be observed. Both hypertrophic and dilated cardiomyopathy have been described. Arrhythmias are common in individuals with MERRF and can accompany the cardiomyopathy or be an isolated cardiac finding.
Lipomatosis can be seen in adulthood in individuals with MERRF, with an average age of onset of 45.2 years [Chong et al 2003]. Lipomas can be infiltrative, progressive, and massive in size [Gilson & Osswald 2018]. The most common location of lipomas is the cervical region.
Phenotype Correlations by Gene
No phenotype correlations by gene have been identified.
Genotype-Phenotype Correlations
No genotype-phenotype correlations have been identified.
For all mtDNA pathogenic variants, clinical expression depends on three factors:
- Heteroplasmy. The relative abundance of mutated mtDNAs
- Tissue distribution of mutated mtDNAs
- Threshold effect. The vulnerability of each tissue to impaired oxidative metabolism
The tissue vulnerability threshold probably does not vary substantially among individuals, but variable mutational load and tissue distribution may account for the clinical diversity of individuals with MERRF.
Penetrance
See Genotype-Phenotype Correlations.
Nomenclature
Ramsay Hunt [1921] described six individuals with a disorder characterized by ataxia, myoclonus, and epilepsy, which he called "dyssynergia cerebellaris myoclonica." Individuals with the diagnosis of Ramsay Hunt syndrome should be investigated for MERRF.
Prevalence
Four epidemiologic studies of mtDNA-related diseases in northern Europe gave concordantly low estimates for the prevalence of the m.8344A>G pathogenic variant:
- 0-1.5:100,000 in the adult population of northern Finland [Remes et al 2005]
- 0.39:100,000 in the adult population of northern England [Schaefer et al 2008]
- 0-0.25:100,000 in a pediatric population of western Sweden [Darin et al 2001]
- 0.7:100,000 in a large population-based study in northeast England [Gorman et al 2015]
See Mitochondrial Disorders Overview for general prevalence information.
Differential Diagnosis
Neurologic findings. The differential diagnosis includes other mitochondrial disorders (see Mitochondrial Disorders Overview), syndromes characterized by ataxia (see Hereditary Ataxia Overview) and myoclonus epilepsy (e.g., Unverricht-Lundborg disease, Lafora type progressive myoclonus epilepsy, neuronal ceroid lipofuscinosis, and sialidosis [Kälviäinen 2015]), and the disorders summarized in Table 4.
The multisystem involvement, lactic acidosis, evidence of maternal inheritance, and muscle biopsy with RRF (ragged red fibers) distinguish MERRF (myoclonic epilepsy with ragged red fibers) from other conditions.
Table 4.
Genes of Interest in the Differential Diagnosis of MERRF
| Gene(s) | DiffDx Disorder | MOI | Clinical Features of DiffDx Disorder | Distinguishing Features |
|---|---|---|---|---|
| CARS2 | Combined oxidative phosphorylation deficiency 27 (OMIM 616672) | AR | Juvenile-onset MERRF-like severe myoclonus epilepsy w/ataxia, spastic tetraparesis, vision loss, hearing loss, & cognitive decline | AR inheritance |
| MT-ND5 MT-TC | MERRF/MELAS overlap syndrome | Mat | May initially resemble MERRF 1 | Stroke-like episodes |
| MT-TL2 | MT-TL2 disorder | Mat | Features of MERRF & NARP in 1 person 2 | Retinitis pigmentosa |
| POLG | POLG-related disorders | AR | Myoclonus, epilepsy, ataxia, peripheral neuropathy | Absence of RRF in POLG phenotype |
AR = autosomal recessive; DiffDx = differential diagnosis; Mat = maternal; MOI = mode of inheritance; NARP = neuropathy-ataxia-retinitis pigmentosa; RRF = ragged red fibers
- 1.
Crimi et al [2003], DiMauro & Davidzon [2005], Naini et al [2005], Herrero-Martín et al [2010]
- 2.
Martín-Jiménez et al [2012]
Lipomas. Other syndromes that cause multiple lipomas (e.g., multiple symmetric lipomatosis [OMIM 151800]) need to be considered.
Management
Evaluations Following Initial Diagnosis
To establish the extent of disease and needs in an individual diagnosed with MERRF (myoclonic epilepsy with ragged red fibers), the evaluations summarized in Table 5 (if not performed as part of the evaluation that led to the diagnosis) are recommended.
Table 5.
Recommended Evaluations Following Initial Diagnosis in Individuals with MERRF
| System/Concern | Evaluation | Comment |
|---|---|---|
| Growth | Measurement of height & weight | To evaluate for short stature |
| Neurologic | Neurologic eval | To assess for neurologic deficits |
| Head MRI w/MRS | To evaluate for pathologic changes at baseline | |
| EEG | If seizures are suspected | |
| Neuropsychiatric testing | To assess cognitive abilities & evidence of dementia | |
| Ears | Audiologic eval | To detect hearing loss |
| Eyes | Ophthalmologic eval | To screen for ptosis, optic atrophy, pigmentary retinopathy, ophthalmoplegia, vision deficits |
| Musculoskeletal | PT/OT assessment | For persons w/neurologic deficits |
| Cardiovascular | Cardiac eval incl echocardiogram | To evaluate for cardiomyopathy & cardiac defects |
| Electrocardiogram | To screen for conduction abnormalities | |
| Endocrinologic |
| To screen for diabetes mellitus |
| Genetic counseling | By genetics professionals 1 | To inform patients & their families re nature, MOI, & implications of MERRF to facilitate medical & personal decision making |
| Family support/ resources | Assess:
|