Ror2-Related Robinow Syndrome
Summary
Clinical characteristics.
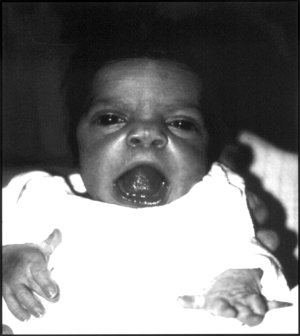
ROR2-related Robinow syndrome is characterized by distinctive craniofacial features, skeletal abnormalities, and other anomalies. Craniofacial features include macrocephaly, broad prominent forehead, low-set ears, ocular hypertelorism, prominent eyes, midface hypoplasia, short upturned nose with depressed nasal bridge and flared nostrils, large and triangular mouth with exposed incisors and upper gums, gum hypertrophy, misaligned teeth, ankyloglossia, and micrognathia. Skeletal abnormalities include short stature, mesomelic or acromesomelic limb shortening, hemivertebrae with fusion of thoracic vertebrae, and brachydactyly. Other common features include micropenis with or without cryptorchidism in males and reduced clitoral size and hypoplasia of the labia majora in females, renal tract abnormalities, and nail hypoplasia or dystrophy. The disorder is recognizable at birth or in early childhood.
Diagnosis/testing.
The diagnosis of ROR2-related Robinow syndrome is established in a proband with typical suggestive findings and biallelic ROR2 pathogenic variants identified on molecular genetic testing.
Management.
Treatment of manifestations: Corrective surgery for limb and spine defects and for facial abnormalities; orthodontic treatment as needed; surgery for males with scrotal transposition as needed; hormone therapy as needed for the treatment of micropenis.
Genetic counseling.
ROR2-related Robinow syndrome is inherited in an autosomal recessive manner. At conception, each sib of an affected individual has a 25% chance of being affected with Robinow syndrome, a 50% chance of being a heterozygote (carrier) and usually asymptomatic, and a 25% chance of being unaffected and not a carrier. Carrier testing for at-risk family members, prenatal diagnosis for pregnancies at increased risk, and preimplantation genetic testing are possible when the ROR2 pathogenic variants have been identified in the family.
Diagnosis
Suggestive Findings
ROR2-related Robinow syndrome should be suspected in individuals with the following clinical findings and family history.
Clinical Findings
Craniofacial findings in early childhood [Tufan et al 2005, Brunetti-Pierri et al 2008]:
- Macrocephaly
- Dysmorphic facial features including: broad prominent forehead, marked ocular hypertelorism, prominent eyes with apparent exophthalmos resulting from deficiency of the lower eyelid (giving the eyes a more prominent appearance), midface hypoplasia, short upturned nose with depressed nasal bridge and flared nostrils, large and triangular mouth (usually with tethering of the upper lip in the center so it appears like an inverted V and exposes the incisors and upper gum), micrognathia, and simple, low-set ears (which can be posteriorly rotated)
- Cleft lip and/or cleft palate
- Crowded and misaligned teeth, gum hypertrophy, and ankyloglossia (with bifid tongue in severe cases)
Skeletal
- Short stature. Birth length is reduced; height was consistently ≥2 SD below the mean in one series [Soliman et al 1998].
- Mesomelic or acromesomelic limb shortening, mostly in the forearms
- Brachydactyly with shortening of the distal phalanx, especially the second and fifth digit; clefting of the distal phalanx of the thumb and occasionally other distal phalanges; variable soft-tissue syndactyly involving two or more digits
- Hemivertebrae with fusion of thoracic vertebrae; ribs usually fused or absent [Patton & Afzal 2002, Tufan et al 2005]
Genital
- In males, micropenis with normal scrotum and testes, or cryptorchidism
- In females, reduced clitoral size and hypoplasia of the labia majora
Family History
Family history is consistent with autosomal recessive inheritance. To date ROR2-related Robinow syndrome has been reported in consanguineous populations as well as in nonconsanguineous populations showing a founder effect (see Aglan et al [2015] for review).
Establishing the Diagnosis
The diagnosis of ROR2-related Robinow syndrome is established in a proband with typical suggestive findings in whom biallelic ROR2 pathogenic variants have been identified by molecular genetic testing (see Table 1).
Molecular genetic testing approaches can include a combination of gene-targeted testing (single-gene testing, multigene panel) and comprehensive genomic testing (exome sequencing, exome array).
Gene-targeted testing requires that the clinician determine which gene(s) are likely involved, whereas genomic testing does not. Because the phenotype of ROR2-related Robinow syndrome is well described, individuals with the distinctive findings described in Suggestive Findings are likely to be diagnosed using gene-targeted testing (see Option 1), whereas those in whom the diagnosis of ROR2-related Robinow syndrome has not been considered are more likely to be diagnosed using genomic testing (see Option 2).
Option 1
When the phenotypic findings and family history suggest the diagnosis of ROR2-related Robinow syndrome, molecular genetic testing approaches can include single-gene testing or use of a multigene panel.
- Single-gene testing. Sequence analysis of ROR2 detects small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. Perform sequence analysis first. If only one or no pathogenic variant is found, perform gene-targeted deletion/duplication analysis to detect intragenic deletions or duplications.
- A multigene panel that includes ROR2 and other genes of interest (see Differential Diagnosis) is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests. For this disorder a multigene panel that also includes deletion/duplication analysis is recommended (see Table 1).For an introduction to multigene panels click here. More detailed information for clinicians ordering genetic tests can be found here.
Option 2
When the diagnosis of ROR2-related Robinow syndrome has not been considered, affected individuals are most likely to be diagnosed using comprehensive genomic testing (which does not require the clinician to determine which gene[s] are likely involved). Exome sequencing is most commonly used; genome sequencing is also possible.
Exome array (when clinically available) may be considered if exome sequencing is not diagnostic.
For an introduction to comprehensive genomic testing click here. More detailed information for clinicians ordering genomic testing can be found here.
Table 1.
Molecular Genetic Testing Used in ROR2-Related Robinow Syndrome
| Gene 1 | Method | Proportion of Pathogenic Variants 2 Detectable by Method |
|---|---|---|
| ROR2 | Sequence analysis 3 | >95% |
| Gene-targeted deletion/duplication analysis 4 | Unknown 5 |
- 1.
See Table A. Genes and Databases for chromosome locus and protein.
- 2.
See Molecular Genetics for information on allelic variants detected in this gene.
- 3.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Pathogenic variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 4.
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications.
- 5.
The frequency of exon or whole-gene deletions and duplications in this disorder is not known; however, exon deletions have been reported [Brunetti-Pierri et al 2008].
Clinical Characteristics
Clinical Description
ROR2-related Robinow syndrome is characterized by distinctive craniofacial features, skeletal abnormalities, and other anomalies.
Craniofacial. Facies are characteristic at birth and in early childhood (see Suggestive Findings). The face in early childhood resembles a fetal face at eight weeks' gestation; this becomes less noticeable with age. Accelerated growth of the nose in adolescence gives the face a more normal appearance, but the broad forehead, broad nasal root, and ocular hypertelorism persist into adulthood.
Midline cleft lip and palate has been reported but is not a common finding. A rather unusual form of clefting involving the lower lip has been described in some individuals.
Dental problems, including wide retromolar ridge, alveolar ridge deformation, malocclusion, dental crowding, and hypodontia, are also common in Robinow syndrome.
Hyperplastic gingival tissues may also interfere with dental eruption and orthodontic treatments [Grothe et al 2008, Beiraghi et al 2011].
Skeletal. Short stature is almost always present in childhood and persists into adulthood; however, the final degree of short stature may be mild [Tufan et al 2005].
The forearms are more noticeably affected by mesomelic or acromesomelic shortening than the lower limbs, often with radioulnar dislocation.
The phalanges and carpal bones may be fused. Partial cutaneous syndactyly or ectrodactyly (i.e., split hand) may be seen. Hand function is not severely affected.
Kyphoscoliosis is often severe. The chest may be deformed and ribs are often fused, as in spondylocostal dysostosis; some ribs may even be absent. Primary lung function is normal, but changes in the chest wall and thoracic vertebrae may reduce cough effort and predispose to respiratory infections [Sleesman & Tobias 2003].
Urogenital. At birth, the genitalia are abnormal, sometimes leading (primarily in males) to issues related to sex assignment. In males, the penis is small; scrotum and testes are normal. Cryptorchidism has been reported. In females, clitoral size is reduced; labia majora may be hypoplastic.
Wilcox et al [1997] determined that in Robinow syndrome the penis is buried inferiorly and posteriorly within the scrotum because the penile crura insert inferiorly and posteriorly onto the medial aspect of the ischial tuberosity (rather than onto the anteromedial aspect of the pubic bone). Thus, a normal-sized penis appears shorter and inferiorly placed in the scrotum.
Endocrine investigations are usually normal; however, Soliman et al [1998] reported low basal serum testosterone concentration and low testosterone response to human chorionic gonadotropin stimulation in boys. Puberty is usually normal.
Renal abnormalities may be associated with the genital abnormalities. Hydronephrosis is common and cystic dysplasia of the kidney has been reported.
Other
- Congenital heart defects are seen in 15%. In addition to pulmonary valve stenosis or atresia, cardiac defects include atrial septal defect, ventricular septal defect, coarctation of the aorta, tetralogy of Fallot, and tricuspid atresia [Al-Ata et al 1998]. Congenital heart defects are the major cause of early death.
- Nail hypoplasia or dystrophy may be present.
- Intellect is usually within the normal range; however, developmental delay has been reported.
Genotype-Phenotype Correlations
No genotype-phenotype correlations are known.
Nomenclature
Other names by which Robinow syndrome has been known in the past:
- Costovertebral segmentation defect with mesomelia (COVESDEM): this name is no longer used because it causes confusion with similar vertebral defect syndromes, and in ROR2-related Robinow syndrome, acromesomelia as well as mesomelia is present.
- Robinow-Silverman syndrome
Prevalence
ROR2-related Robinow syndrome is rare. More than 100 cases have been reported in the literature. It commonly occurs in consanguineous families; for example, those of Turkish and Omani origin.
Differential Diagnosis
NXN-related Robinow syndrome (OMIM 618529), an autosomal recessive form of Robinow syndrome, was described in three individuals with biallelic NXN pathogenic variants from two unrelated families. All three had classic clinical findings of Robinow syndrome including typical craniofacial features, mesomelic shortening, and brachydactyly [White et al 2018]. One individual, born to consanguineous parents, was homozygous for a nonsense NXN variant; the two affected sibs in the other family had compound heterozygous NXN pathogenic variants.
Note: The NXN protein is a relevant partner in the WNT5A signaling pathway that is intimately involved in Robinow syndrome causation. ROR2 binds to WNT5A and interacts with FZD2. The effect of this interaction is routed to disheveled proteins (DVL1, DVL3) that are further stabilized by NXN. This complex activates JNK signaling responsible for cytoskeletal reorganization and cell polarity.
Biallelic WNT5A pathogenic variants were identified in one individual with findings similar to those of ROR2-related Robinow syndrome [Author, unpublished observation].
Autosomal dominant Robinow syndrome, described by Robinow et al [1969], is similar to ROR2-related Robinow syndrome, but has more severe dental anomalies and less severe skeletal defects:
- Vertebral anomalies and radial head dislocation are rare [Bain et al 1986].
- Vertebral anomalies and scoliosis are seen in far fewer of those with AD Robinow syndrome (<25%) compared to those with AR Robinow syndrome (>75%) [Mazzeu et al 2007].
- Height is usually nearer the normal range in AD Robinow syndrome.
Autosomal dominant Robinow syndrome is rarer than autosomal recessive Robinow syndrome.
The diagnosis of autosomal dominant Robinow syndrome is established in a proband with typical suggestive findings and/or by the identification of a heterozygous pathogenic variant in DVL1, DVL3, or WNT5A through molecular genetic testing. WNT5A is a known coreceptor of the tyrosine kinase receptor, ROR2, which would explain the overlap in clinical phenotype of WNT5A- and ROR2-associated Robinow syndrome.
Jarcho-Levin syndrome and spondylocostal dysostosis (see Spondylocostal Dysostosis, Autosomal Recessive) are diagnosed radiologically and show vertebral and rib abnormalities similar to those found in ROR2-related Robinow syndrome; short trunk and respiratory insufficiency are present.
I-cell disease (mucolipidosis type II) is a lysosomal storage disorder showing growth failure, coarse facial features, hypertrophic gums, skeletal abnormalities, developmental delay, and hypotonia.
Aarskog syndrome (OMIM 100050). Facial features are similar to those in ROR2-related Robinow syndrome: wide-spaced eyes, anteverted nostrils, and broad upper lip. Vertebral abnormalities are not observed. The shawl scrotum and lax ligaments of Aarskog syndrome are not found in ROR2-related Robinow syndrome.
Omodysplasia (OMIM 258315 and 164745) is similar to ROR2-related Robinow syndrome, with short limbs and radial dislocation; however, no genital abnormalities are present [Venditti et al 2002].
Management
Evaluations Following Initial Diagnosis
To establish the extent of disease and needs in an individual diagnosed with ROR2-related Robinow syndrome, the evaluations summarized in this section (if not performed as part of the evaluation that led to the diagnosis) are recommended:
- Clinical assessment for presence of cleft lip/palate and the need for surgical repair
- Orthodontics consultation as needed for misaligned, crowded teeth
- Clinical and radiographic evaluation of the spine and rib cage to assess the severity of kyphoscoliosis and vertebral and rib anomalies, as these can lead to postural and respiratory complications [Wilcox et al 1997]
- Radiographic documentation of radioulnar synostosis, forearm shortening, and brachydactyly
- Urology consultation in males with cryptorchidism and abnormal penile insertion / penoscrotal transposition for consideration of reconstructive surgery
- Endocrine consultation to assess the possibility of hormone therapy for males with micropenis
- Renal ultrasound examination
- Echocardiogram to evaluate for structural heart defects
- Consultation with a clinical geneticist and/or genetic counselor
Treatment of Manifestations
Corrective surgeries may be required for the following:
- Syndactyly
- Severe scoliosis secondary to hemivertebrae and rib abnormalities
- Cleft lip/palate
- Abnormal penile insertion / penoscrotal transposition. Although it was not possible to detach the abnormal insertion of the penile crura, which can cause a normal-sized penis to be buried in the scrotum and thus appear small, Wilcox et al [1997] improved the cosmetic appearance by transposing the scrotum downward.
Injection of human chorionic gonadotropin and testosterone therapy improved penile length and testicular volume in three boys with severe micropenis [Soliman et al 1998]. Hormone therapy should be monitored by a pediatric endocrinologist.
Orthodontic treatment is usually required.
Perioperative management of individuals with Robinow syndrome should include the following [Macdonald & Dearlove 1995, Lirk et al 2003, Sleesman & Tobias 2003]:
- Preoperative radiologic assessment of the vertebrae and ribs because of the risk for respiratory complications
- Preoperative cardiac evaluation for the presence of congenital heart defects
- Awareness that endotracheal intubation may be difficult as a result of midface hypoplasia
Surveillance
The following are appropriate:
- Surveillance for evidence of scoliosis until growth is completed
- Dental evaluation every six months to one year or as recommended by the dental professional on initial assessment
- Regular cardiac and renal assessment by respective specialists as needed if abnormalities are identified
Evaluation of Relatives at Risk
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Therapies Under Investigation
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for information on clinical studies for a wide range of diseases and conditions. Note: There may not be clinical trials for this disorder.