Carbon Monoxide Poisoning

Carbon monoxide poisoning typically occurs from breathing in carbon monoxide (CO) at excessive levels. Symptoms are often described as "flu-like" and commonly include headache, dizziness, weakness, vomiting, chest pain, and confusion. Large exposures can result in loss of consciousness, arrhythmias, seizures, or death. The classically described "cherry red skin" rarely occurs. Long-term complications may include feeling tired, trouble with memory, and movement problems. In those exposed to smoke, cyanide toxicity should also be considered.
Carbon monoxide poisoning can occur accidentally, as an attempt to end one's own life, or as an attempt to end another's life. CO is a colorless and odorless gas which is initially non-irritating. It is produced during incomplete burning of organic matter. This can occur from motor vehicles, heaters, or cooking equipment that run on carbon-based fuels. It can also occur from exposure to methylene chloride. Carbon monoxide primarily causes adverse effects by combining with hemoglobin to form carboxyhemoglobin (HbCO) preventing the blood from carrying oxygen. Additionally, myoglobin and mitochondrial cytochrome oxidase are affected. Diagnosis is based on a HbCO level of more than 3% among nonsmokers and more than 10% among smokers.
Efforts to prevent poisoning include carbon monoxide detectors, proper venting of gas appliances, keeping chimneys clean, and keeping exhaust systems of vehicles in good repair. Treatment of poisoning generally consists of giving 100% oxygen along with supportive care. This should generally be carried out until symptoms are no longer present and the HbCO level is less than 10%. While hyperbaric oxygen therapy is used for severe poisonings, the benefit over standard oxygen delivery is unclear. The risk of death among those affected is between 1 and 30%.
Carbon monoxide poisoning is relatively common, resulting in more than 20,000 emergency room visits a year in the United States. It is the most common type of fatal poisoning in many countries. In the United States, non-fire related cases result in more than 400 deaths a year. Poisonings occur more often in the winter, particularly from the use of portable generators during power outages. The toxic effects of CO have been known since ancient history. The discovery that hemoglobin is affected by CO was made in 1857.
Signs and symptoms
Carbon monoxide is not toxic to all forms of life. Its harmful effects are due to binding with hemoglobin so its danger to organisms that do not use this compound is doubtful. It thus has no effect on photosynthesising plants. It is easily absorbed through the lungs. Inhaling the gas can lead to hypoxic injury, nervous system damage, and even death. Different people and populations may have different carbon monoxide tolerance levels. On average, exposures at 100 ppm or greater is dangerous to human health. In the United States, the OSHA limits long-term workplace exposure levels to less than 50 ppm averaged over an 8-hour period; in addition, employees are to be removed from any confined space if an upper limit ("ceiling") of 100 ppm is reached. Carbon monoxide exposure may lead to a significantly shorter life span due to heart damage. The carbon monoxide tolerance level for any person is altered by several factors, including activity level, rate of ventilation, a pre-existing cerebral or cardiovascular disease, cardiac output, anemia, sickle cell disease and other hematological disorders, barometric pressure, and metabolic rate.
| Concentration | Symptoms |
|---|---|
| 35 ppm (0.0035%), (0.035‰) | Headache and dizziness within six to eight hours of constant exposure |
| 100 ppm (0.01%), (0.1‰) | Slight headache in two to three hours |
| 200 ppm (0.02%), (0.2‰) | Slight headache within two to three hours; loss of judgment |
| 400 ppm (0.04%), (0.4‰) | Frontal headache within one to two hours |
| 800 ppm (0.08%), (0.8‰) | Dizziness, nausea, and convulsions within 45 min; insensible within 2 hours |
| 1,600 ppm (0.16%), (1.6‰) | Headache, increased heart rate, dizziness, and nausea within 20 min; death in less than 2 hours |
| 3,200 ppm (0.32%), (3.2‰) | Headache, dizziness and nausea in five to ten minutes. Death within 30 minutes. |
| 6,400 ppm (0.64%), (6.4‰) | Headache and dizziness in one to two minutes. Convulsions, respiratory arrest, and death in less than 20 minutes. |
| 12,800 ppm (1.28%), (12.8‰) | Unconsciousness after 2–3 breaths. Death in less than three minutes. |
Acute poisoning

The main manifestations of carbon monoxide poisoning develop in the organ systems most dependent on oxygen use, the central nervous system and the heart. The initial symptoms of acute carbon monoxide poisoning include headache, nausea, malaise, and fatigue. These symptoms are often mistaken for a virus such as influenza or other illnesses such as food poisoning or gastroenteritis. Headache is the most common symptom of acute carbon monoxide poisoning; it is often described as dull, frontal, and continuous. Increasing exposure produces cardiac abnormalities including fast heart rate, low blood pressure, and cardiac arrhythmia; central nervous system symptoms include delirium, hallucinations, dizziness, unsteady gait, confusion, seizures, central nervous system depression, unconsciousness, respiratory arrest, and death. Less common symptoms of acute carbon monoxide poisoning include myocardial ischemia, atrial fibrillation, pneumonia, pulmonary edema, high blood sugar, lactic acidosis, muscle necrosis, acute kidney failure, skin lesions, and visual and auditory problems.
One of the major concerns following acute carbon monoxide poisoning is the severe delayed neurological manifestations that may occur. Problems may include difficulty with higher intellectual functions, short-term memory loss, dementia, amnesia, psychosis, irritability, a strange gait, speech disturbances, Parkinson's disease-like syndromes, cortical blindness, and a depressed mood. Depression may occur in those who did not have pre-existing depression. These delayed neurological sequelae may occur in up to 50% of poisoned people after 2 to 40 days. It is difficult to predict who will develop delayed sequelae; however, advanced age, loss of consciousness while poisoned, and initial neurological abnormalities may increase the chance of developing delayed symptoms.
One classic sign of carbon monoxide poisoning is more often seen in the dead rather than the living – people have been described as looking red-cheeked and healthy (see below). However, since this "cherry-red" appearance is more common in the dead, it is not considered a useful diagnostic sign in clinical medicine. In autopsy examinations, the ruddy appearance of carbon monoxide poisoning is notable because unembalmed dead persons are normally bluish and pale, whereas dead carbon-monoxide poisoned people may appear unusually lifelike in coloration. The colorant effect of carbon monoxide in such postmortem circumstances is thus analogous to its use as a red colorant in the commercial meat-packing industry.
Chronic poisoning
Chronic exposure to relatively low levels of carbon monoxide may cause persistent headaches, lightheadedness, depression, confusion, memory loss, nausea, hearing disorders and vomiting. It is unknown whether low-level chronic exposure may cause permanent neurological damage. Typically, upon removal from exposure to carbon monoxide, symptoms usually resolve themselves, unless there has been an episode of severe acute poisoning. However, one case noted permanent memory loss and learning problems after a three-year exposure to relatively low levels of carbon monoxide from a faulty furnace. Chronic exposure may worsen cardiovascular symptoms in some people. Chronic carbon monoxide exposure might increase the risk of developing atherosclerosis. Long-term exposures to carbon monoxide present the greatest risk to persons with coronary heart disease and in females who are pregnant. In experimental animals, carbon monoxide appears to worsen noise-induced hearing loss at noise exposure conditions that would have limited effects on hearing otherwise. In humans, hearing loss has been reported following carbon monoxide poisoning. Unlike the findings in animal studies, noise exposure was not a necessary factor for the auditory problems to occur.
Causes
| Concentration | Source |
|---|---|
| 0.1 ppm | Natural atmosphere level (MOPITT) |
| 0.5 to 5 ppm | Average level in homes |
| 5 to 15 ppm | Near properly adjusted gas stoves in homes |
| 100 to 200 ppm | Exhaust from automobiles in the Mexico City central area |
| 5,000 ppm | Exhaust from a home wood fire |
| 7,000 ppm | Undiluted warm car exhaust without a catalytic converter |
| 30,000 ppm | Afterdamp following an explosion in a coal mine |
Carbon monoxide is a product of combustion of organic matter under conditions of restricted oxygen supply, which prevents complete oxidation to carbon dioxide (CO2). Sources of carbon monoxide include cigarette smoke, house fires, faulty furnaces, heaters, wood-burning stoves, internal combustion vehicle exhaust, electrical generators, propane-fueled equipment such as portable stoves, and gasoline-powered tools such as leaf blowers, lawn mowers, high-pressure washers, concrete cutting saws, power trowels, and welders. Exposure typically occurs when equipment is used in buildings or semi-enclosed spaces.
Riding in the back of pickup trucks has led to poisoning in children. Idling automobiles with the exhaust pipe blocked by snow has led to the poisoning of car occupants. Any perforation between the exhaust manifold and shroud can result in exhaust gases reaching the cabin. Generators and propulsion engines on boats, especially houseboats, has resulted in fatal carbon monoxide exposures.
Poisoning may also occur following the use of a self-contained underwater breathing apparatus (SCUBA) due to faulty diving air compressors.
In caves carbon monoxide can build up in enclosed chambers due to the presence of decomposing organic matter. In coal mines incomplete combustion may occur during explosions resulting in the production of afterdamp. The gas is up to 3% CO and may be fatal after just a single breath. Following an explosion in a colliery, adjacent interconnected mines may become dangerous due to the afterdamp leaking from mine to mine. Such an incident followed the Trimdon Grange explosion which killed men in the Kelloe mine.
Another source of poisoning is exposure to the organic solvent dichloromethane, also known as methylene chloride, found in some paint strippers, as the metabolism of dichloromethane produces carbon monoxide. In November 2019, an EPA ban on dichloromethane in paint strippers for consumer use took effect in the United States.
Pathophysiology
The precise mechanisms by which the effects of carbon monoxide are induced upon bodily systems, are complex and not yet fully understood. Known mechanisms include carbon monoxide binding to hemoglobin, myoglobin and mitochondrial cytochrome c oxidase and restricting oxygen supply, and carbon monoxide causing brain lipid peroxidation.
Hemoglobin
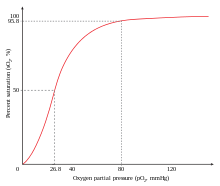
Carbon monoxide has a higher diffusion coefficient compared to oxygen, and the only enzyme in the human body that produces carbon monoxide is heme oxygenase, which is located in all cells and breaks down heme. Under normal conditions, carbon monoxide levels in the plasma are approximately 0 mmHg because it has a higher diffusion coefficient and the body easily gets rid of any CO made. When CO is not ventilated it binds to hemoglobin, which is the principal oxygen-carrying compound in blood; this produces a compound known as carboxyhemoglobin. The traditional understanding is that carbon monoxide toxicity arises from the formation of carboxyhemoglobin, which decreases the oxygen-carrying capacity of the blood and inhibits the transport, delivery, and utilization of oxygen by the body. The affinity between hemoglobin and carbon monoxide is approximately 230 times stronger than the affinity between hemoglobin and oxygen so hemoglobin binds to carbon monoxide in preference to oxygen.
Hemoglobin is a tetramer with four oxygen binding sites. The binding of carbon monoxide at one of these sites increases the oxygen affinity of the remaining three sites, which causes the hemoglobin molecule to retain oxygen that would otherwise be delivered to the tissue. This situation is described as carbon monoxide shifting the oxygen dissociation curve to the left. Because of the increased affinity between hemoglobin and oxygen during carbon monoxide poisoning, little oxygen will actually be released in the tissues. This causes hypoxic tissue injury. Hemoglobin acquires a bright red color when converted into carboxyhemoglobin, so poisoned cadavers and even commercial meats treated with carbon monoxide acquire an unnatural reddish hue.
Myoglobin
Carbon monoxide also binds to the hemeprotein myoglobin. It has a high affinity for myoglobin, about 60 times greater than that of oxygen. Carbon monoxide bound to myoglobin may impair its ability to utilize oxygen. This causes reduced cardiac output and hypotension, which may result in brain ischemia. A delayed return of symptoms have been reported. This results following a recurrence of increased carboxyhemoglobin levels; this effect may be due to a late release of carbon monoxide from myoglobin, which subsequently binds to hemoglobin.
Cytochrome oxidase
Another mechanism involves effects on the mitochondrial respiratory enzyme chain that is responsible for effective tissue utilization of oxygen. Carbon monoxide binds to cytochrome oxidase with less affinity than oxygen, so it is possible that it requires significant intracellular hypoxia before binding. This binding interferes with aerobic metabolism and efficient adenosine triphosphate synthesis. Cells respond by switching to anaerobic metabolism, causing anoxia, lactic acidosis, and eventual cell death. The rate of dissociation between carbon monoxide and cytochrome oxidase is slow, causing a relatively prolonged impairment of oxidative metabolism.
Central nervous system effects
The mechanism that is thought to have a significant influence on delayed effects involves formed blood cells and chemical mediators, which cause brain lipid peroxidation (degradation of unsaturated fatty acids). Carbon monoxide causes endothelial cell and platelet release of nitric oxide, and the formation of oxygen free radicals including peroxynitrite. In the brain this causes further mitochondrial dysfunction, capillary leakage, leukocyte sequestration, and apoptosis. The result of these effects is lipid peroxidation, which causes delayed reversible demyelination of white matter in the central nervous system known as Grinker myelinopathy, which can lead to edema and necrosis within the brain. This brain damage occurs mainly during the recovery period. This may result in cognitive defects, especially affecting memory and learning, and movement disorders. These disorders are typically related to damage to the cerebral white matter and basal ganglia. Hallmark pathological changes following poisoning are bilateral necrosis of the white matter, globus pallidus, cerebellum, hippocampus and the cerebral cortex.
Pregnancy
Carbon monoxide poisoning in pregnant women may cause severe adverse fetal effects. Poisoning causes fetal tissue hypoxia by decreasing the release of maternal oxygen to the fetus. Carbon monoxide also crosses the placenta and combines with fetal hemoglobin, causing more direct fetal tissue hypoxia. Additionally, fetal hemoglobin has a 10 to 15% higher affinity for carbon monoxide than adult hemoglobin, causing more severe poisoning in the fetus than in the adult. Elimination of carbon monoxide is slower in the fetus, leading to an accumulation of the toxic chemical. The level of fetal morbidity and mortality in acute carbon monoxide poisoning is significant, so despite mild maternal poisoning or following maternal recovery, severe fetal poisoning or death may still occur.
Diagnosis

As many symptoms of carbon monoxide poisoning also occur with many other types of poisonings and infections (such as the flu), the diagnosis is often difficult. A history of potential carbon monoxide exposure, such as being exposed to a residential fire, may suggest poisoning, but the diagnosis is confirmed by measuring the levels of carbon monoxide in the blood. This can be determined by measuring the amount of carboxyhemoglobin compared to the amount of hemoglobin in the blood.
The ratio of carboxyhemoglobin to hemoglobin molecules in an average person may be up to 5%, although cigarette smokers who smoke two packs per day may have levels up to 9%. In symptomatic poisoned people they are often in the 10–30% range, while persons who die may have postmortem blood levels of 30–90%.
As people may continue to experience significant symptoms of CO poisoning long after their blood carboxyhemoglobin concentration has returned to normal, presenting to examination with a normal carboxyhemoglobin level (which may happen in late states of poisoning) does not rule out poisoning.
Measuring
Carbon monoxide may be quantitated in blood using spectrophotometric methods or chromatographic techniques in order to confirm a diagnosis of poisoning in a person or to assist in the forensic investigation of a case of fatal exposure.
A CO-oximeter can be used to determine carboxyhemoglobin levels. Pulse CO-oximeters estimate carboxyhemoglobin with a non-invasive finger clip similar to a pulse oximeter. These devices function by passing various wavelengths of light through the fingertip and measuring the light absorption of the different types of hemoglobin in the capillaries. The use of a regular pulse oximeter is not effective in the diagnosis of carbon monoxide poisoning as people with carbon monoxide poisoning may have a normal oxygen saturation level on a pulse oximeter. This is due to the carboxyhemoglobin being misrepresented as oxyhemoglobin.
Breath CO monitoring offers an alternative to pulse CO-oximetry. Carboxyhemoglobin levels have been shown to have a strong correlation with breath CO concentration. However, many of these devices require the user to inhale deeply and hold their breath to allow the CO in the blood to escape into the lung before the measurement can be made. As this is not possible in people who are unresponsive, these devices may not appropriate for use in on-scene emergency care detection of CO poisoning.
Differential diagnosis
There are many conditions to be considered in the differential diagnosis of carbon monoxide poisoning. The earliest symptoms, especially from low level exposures, are often non-specific and readily confused with other illnesses, typically flu-like viral syndromes, depression, chronic fatigue syndrome, chest pain, and migraine or other headaches. Carbon monoxide has been called a "great mimicker" due to the presentation of poisoning being diverse and nonspecific. Other conditions included in the differential diagnosis include acute respiratory distress syndrome, altitude sickness, lactic acidosis, diabetic ketoacidosis, meningitis, methemoglobinemia, or opioid or toxic alcohol poisoning.
Prevention
Detectors

Prevention remains a vital public health issue, requiring public education on the safe operation of appliances, heaters, fireplaces, and internal-combustion engines, as well as increased emphasis on the installation of carbon monoxide detectors. Carbon monoxide is tasteless, odourless, and colourless, and therefore can not be detected by visual cues or smell.
The United States Consumer Product Safety Commission has stated, "carbon monoxide detectors are as important to home safety as smoke detectors are," and recommends each home have at least one carbon monoxide detector, and preferably one on each level of the building. These devices, which are relatively inexpensive and widely available, are either battery- or AC-powered, with or without battery backup. In buildings, carbon monoxide detectors are usually installed around heaters and other equipment. If a relatively high level of carbon monoxide is detected, the device sounds an alarm, giving people the chance to evacuate and ventilate the building. Unlike smoke detectors, carbon monoxide detectors do not need to be placed near ceiling level.
The use of carbon monoxide detectors has been standardized in many areas. In the US, NFPA 720-2009, the carbon monoxide detector guidelines published by the National Fire Protection Association, mandates the placement of carbon monoxide detectors/alarms on every level of the residence, including the basement, in addition to outside sleeping areas. In new homes, AC-powered detectors must have battery backup and be interconnected to ensure early warning of occupants at all levels. NFPA 720-2009 is the first national carbon monoxide standard to address devices in non-residential buildings. These guidelines, which now pertain to schools, healthcare centers, nursing homes and other non-residential buildings, include three main points:
- 1. A secondary power supply (battery backup) must operate all carbon monoxide notification appliances for at least 12 hours,
- 2. Detectors must be on the ceiling in the same room as permanently installed fuel-burning appliances, and
- 3. Detectors must be located on every habitable level and in every HVAC zone of the building.
Gas organizations will often recommend to get gas appliances serviced at least once a year.
Legal requirements
The NFPA standard is not necessarily enforced by law. As of April 2006, the US state of Massachusetts requires detectors to be present in all residences with potential CO sources, regardless of building age and whether they are owner-occupied or rented. This is enforced by municipal inspectors, and was inspired by the death of 7-year-old Nicole Garofalo in 2005 due to snow blocking a home heating vent. Other jurisdictions may have no requirement or only mandate detectors for new construction or at time of sale.
Despite similar deaths in vehicles with clogged exhaust pipes (for example in the Northeastern United States blizzard of 1978 and February 2013 nor'easter) and the commercial availability of the equipment, there is no legal requirement for automotive CO detectors.
World Health Organization recommendations
The following guideline values (ppm values rounded) and periods of time-weighted average exposures have been determined in such a way that the carboxyhaemoglobin (COHb) level of 2.5% is not exceeded, even when a normal subject engages in light or moderate exercise:
- 100 mg/m3 (87 ppm) for 15 min
- 60 mg/m3 (52 ppm) for 30 min
- 30 mg/m3 (26 ppm) for 1 h
- 10 mg/m3 (9 ppm) for 8 h
- 7 mg/m3 (6 ppm) for 24 h (for indoor air quality, so as not to exceed 2% COHb for chronic exposure)
Treatment
| Oxygen pressure О2 | Time |
|---|---|
| 21% oxygen at normal atmospheric pressure (fresh air) | 5 hours 20 min |
| 100% oxygen at normal atmospheric pressure (non-rebreather oxygen mask) | 1 hours 20 min |
| 100% hyperbaric oxygen (3 atmospheres absolute) | 23 min |
Initial treatment for carbon monoxide poisoning is to immediately remove the person from the exposure without endangering further people. Those who are unconscious may require CPR on site. Administering oxygen via non-rebreather mask shortens the half-life of carbon monoxide from 320 minutes, when breathing normal air, to only 80 minutes. Oxygen hastens the dissociation of carbon monoxide from carboxyhemoglobin, thus turning it back into hemoglobin. Due to the possible severe effects in the baby, pregnant women are treated with oxygen for longer periods of time than non-pregnant people.
Hyperbaric oxygen
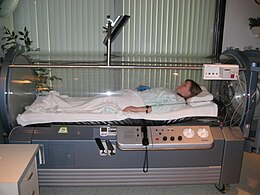
Hyperbaric oxygen is also used in the treatment of carbon monoxide poisoning, as it may hasten dissociation of CO from carboxyhemoglobin and cytochrome oxidase to a greater extent than normal oxygen. Hyperbaric oxygen at three times atmospheric pressure reduces the half life of carbon monoxide to 23 (~80/3 minutes) minutes, compared to 80 minutes for oxygen at regular atmospheric pressure. It may also enhance oxygen transport to the tissues by plasma, partially bypassing the normal transfer through hemoglobin. However, it is controversial whether hyperbaric oxygen actually offers any extra benefits over normal high flow oxygen, in terms of increased survival or improved long-term outcomes. There have been randomized controlled trials in which the two treatment options have been compared; of the six performed, four found hyperbaric oxygen improved outcome and two found no benefit for hyperbaric oxygen. Some of these trials have been criticized for apparent flaws in their implementation. A review of all the literature concluded that the role of hyperbaric oxygen is unclear and the available evidence neither confirms nor denies a medically meaningful benefit. The authors suggested a large, well designed, externally audited, multicentre trial to compare normal oxygen with hyperbaric oxygen.
Other
Further treatment for other complications such as seizure, hypotension, cardiac abnormalities, pulmonary edema, and acidosis may be required. Increased muscle activity and seizures should be treated with dantrolene or diazepam; diazepam should only be given with appropriate respiratory support. Hypotension requires treatment with intravenous fluids; vasopressors may be required to treat myocardial depression. Cardiac dysrhythmias are treated with standard advanced cardiac life support protocols. If severe, metabolic acidosis is treated with sodium bicarbonate. Treatment with sodium bicarbonate is controversial as acidosis may increase tissue oxygen availability. Treatment of acidosis may only need to consist of oxygen therapy. The delayed development of neuropsychiatric impairment is one of the most serious complications of carbon monoxide poisoning. Brain damage is confirmed following MRI or CAT scans. Extensive follow up and supportive treatment is often required for delayed neurological damage. Outcomes are often difficult to predict following poisoning, especially people who have symptoms of cardiac arrest, coma, metabolic acidosis, or have high carboxyhemoglobin levels. One study reported that approximately 30% of people with severe carbon monoxide poisoning will have a fatal outcome. It has been reported that electroconvulsive therapy (ECT) may increase the likelihood of delayed neuropsychiatric sequelae (DNS) after carbon monoxide (CO) poisoning. A device that also provides some carbon dioxide to stimulate faster breathing (sold under the brand name ClearMate) may also be used.
Epidemiology
The true number of cases of carbon monoxide poisoning is unknown, since many non-lethal exposures go undetected. From the available data, carbon monoxide poisoning is the most common cause of injury and death due to poisoning worldwide. Poisoning is typically more common during the winter months. This is due to increased domestic use of gas furnaces, gas or kerosene space heaters, and kitchen stoves during the winter months, which if faulty and/or used without adequate ventilation, may produce excessive carbon monoxide. Carbon monoxide detection and poisoning also increases during power outages, when electric heating and cooking appliances become inoperative and residents may temporarily resort to fuel-burning space heaters, stoves, and grills (some of which are safe only for outdoor use but nonetheless are errantly burned indoors).
It has been estimated that more than 40,000 people per year seek medical attention for carbon monoxide poisoning in the United States. 95% of carbon monoxide poisoning deaths in the United States are due to gas space heaters. In many industrialized countries carbon monoxide is the cause of more than 50% of fatal poisonings. In the United States, approximately 200 people die each year from carbon monoxide poisoning associated with home fuel-burning heating equipment. Carbon monoxide poisoning contributes to the approximately 5613 smoke inhalation deaths each year in the United States. The CDC reports, "Each year, more than 500 Americans die from unintentional carbon monoxide poisoning, and more than 2,000 commit suicide by intentionally poisoning themselves." For the 10-year period from 1979 to 1988, 56,133 deaths from carbon monoxide poisoning occurred in the United States, with 25,889 of those being suicides, leaving 30,244 unintentional deaths. A report from New Zealand showed that 206 people died from carbon monoxide poisoning in the years of 2001 and 2002. In total carbon monoxide poisoning was responsible for 43.9% of deaths by poisoning in that country. In South Korea, 1,950 people had been poisoned by carbon monoxide with 254 deaths from 2001 through 2003. A report from Jerusalem showed 3.53 per 100,000 people were poisoned annually from 2001 through 2006. In Hubei, China, 218 deaths from poisoning were reported over a 10-year period with 16.5% being from carbon monoxide exposure.
History
The earliest description of carbon monoxide poisoning dates to at least 200 BC by Aristotle. Documented cases of carbon monoxide being used as a method of suicide date to at least 100 BC in ancient Rome. In the AD 350s, the Roman emperor Julian suffered from carbon monoxide poisoning in Paris, and later described it in his work Misopogon: "though the winter weather prevailed and continually increased in severity, even so I did not allow my servants to heat the house, because I was afraid of drawing out the dampness in the walls; but I ordered them to carry in fire that had burned down and to place in the room a very moderate number of hot coals. But the coals, though there were not very many of them, brought out from the walls quantities of steam and this made me fall asleep. And since my head was filled with the fumes I was almost choked. Then I was carried outside." This misunderstanding of the causes of carbon monoxide poisoning may have caused the death of Julian's successor, Jovian.
John Scott Haldane identified carbon monoxide as the lethal constituent of afterdamp, the gas created by combustion, after examining many bodies of miners killed in pit explosions. Their skin was coloured cherry-pink from carboxyhaemoglobin, the stable compound formed in the blood by reaction with the gas. As a result of his research, he was able to design respirators for rescue workers. He tested the effect of carbon monoxide on his own body in a closed chamber, describing the results of his slow poisoning. In the late 1890s, he introduced the use of small animals for miners to detect dangerous levels of carbon monoxide underground, either white mice or canaries. With a faster metabolism, they showed the effects of poisoning before gas levels became critical for the workers, and so gave an early warning of the problem. The canary in British pits was replaced in 1986 by the electronic gas detector.
As part of the Holocaust during World War II, German Nazis used gas vans at Chelmno extermination camp and elsewhere to kill an estimated over 700,000 prisoners by carbon monoxide poisoning. This method was also used in the gas chambers of several death camps such as Treblinka, Sobibor and Belzec. Gassing with carbon monoxide started in action T4, the programme developed by the Nazis in Germany to murder the mentally ill and disabled people before the war started in earnest. The gas was supplied by IG Farben in pressurized cylinders and fed by tubes into the gas chambers built at various mental hospitals, such as Hartheim Euthanasia Centre. Many key personnel were recruited from the T4 programme to murder much larger numbers of people in the gas vans and the special gas chambers used in the death camps such as Treblinka. Exhaust fumes from tank engines for example, were used to supply the gas to the chambers.
The worst accidental mass poisoning from carbon monoxide was the Balvano train disaster which occurred on 3 March 1944 in Italy, when a freight train with many illegal passengers stalled in a tunnel, leading to the death of over 500 people.
The use of oxygen as treatment began in 1868. The use of hyperbaric oxygen in rats following poisoning was studied by Haldane in 1895 while its use in humans began in the 1960s.
Research
Carbon monoxide is produced naturally by the body as a byproduct of converting protoporphyrin into bilirubin. This carbon monoxide also combines with hemoglobin to make carboxyhemoglobin, but not at toxic levels.
Small amounts of CO are beneficial and enzymes exist that produce it at times of oxidative stress. Drugs are being developed to introduce small amounts of CO during certain kinds of surgery, these drugs are called carbon monoxide-releasing molecules.