Aortic Aneurysm, Familial Thoracic 1
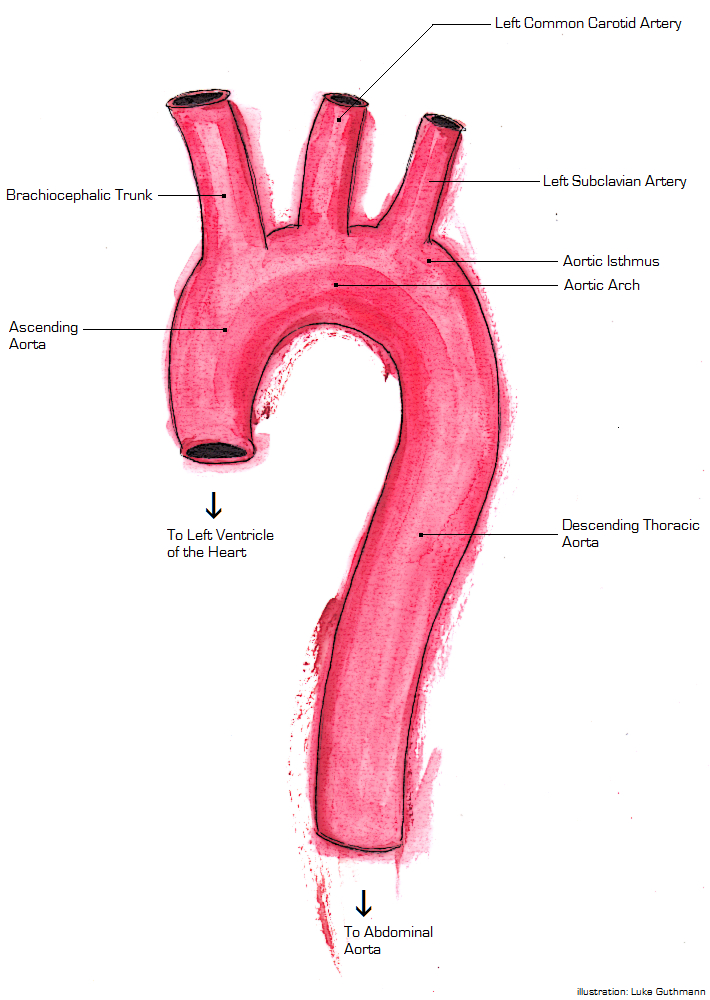
Description
Aneurysms and dissections of the aorta usually result from degenerative changes in the aortic wall. Thoracic aortic aneurysms and dissections are primarily associated with a characteristic histologic appearance known as 'medial necrosis' or 'Erdheim cystic medial necrosis' in which there is degeneration and fragmentation of elastic fibers, loss of smooth muscle cells, and an accumulation of basophilic ground substance. In contrast, degeneration leading to abdominal aortic aneurysm (100070) is usually caused by a combination of factors including age, atherosclerosis, hypertension, and infectious, inflammatory, or autoimmune processes.
Medial necrosis and thoracic aortic aneurysm/dissection are known to occur in certain connective tissue diseases such as Marfan syndrome (154700), and vascular (type IV) Ehlers-Danlos syndrome (130050). More commonly, however, medial necrosis occurs in the absence of a clearly identifiable syndrome.
Genetic Heterogeneity of Thoracic Aortic Aneurysm
Loci for isolated thoracic aortic aneurysm have been identified on chromosomes 11q (AAT1) and 5q (AAT2; 607087). Mutation in the MYH11 gene (160745) on chromosome 16p causes AAT4 (132900). Mutation in the ACTA2 gene (102620) on chromosome 10q causes AAT6 (611788). Mutation in the MYLK gene (600922) on chromosome 3q21 causes AAT7 (613780). Mutation in the PRKG1 gene (176894) on chromosome 10q11 causes AAT8 (615436). Mutation in the MFAP5 gene (601103) on chromosome 12p13 causes AAT9 (616166). Mutation in the LOX gene (153455) on chromosome 5q23 causes AAT10 (617168). Mutation in the FOXE3 gene (601094) on chromosome 1p33 causes susceptibility to AAT11 (617349).
Thoracic aortic aneurysm with dissection (e.g., AAT3 and AAT5) can occur as a manifestation of the Loeys-Dietz syndrome (see LDS2, 610168 and LDS1, 609192, caused by mutation in the TGFBR2 (190182) and TGFBR1 (190181) genes, respectively).
Reviews
Pyeritz (2014) reviewed heritable thoracic aortic disorders with particular attention to causative genes, including components of the extracellular matrix, vascular smooth muscle cytoskeleton, and TGF-beta and other signaling pathways.
Clinical FeaturesMcKusick (1972) noted the association between congenital bicuspid aortic valve and medial necrosis of the aorta in a father and son. Gale et al. (1977) observed 2 brothers with calcific stenosis of a congenital bicuspid aortic valve. One brother had a large aneurysm of the ascending aorta. The second had moderate dilatation. The association of aortic stenosis and cystic medial necrosis was documented by McKusick et al. (1957). Lindsay (1988) reviewed the evidence for an interrelationship between coarctation of the aorta, bicuspid aortic valve, and abnormal ascending aortic wall. The correlation between the first 2 had been pointed out by Abbott (1928). By echocardiographic study, Pachulski et al. (1991) demonstrated increased mean aortic root diameter in patients with functionally normal or minimally stenotic bicuspid aortic valves.
Bixler and Antley (1976) reported a possibly distinct entity combining Erdheim cystic medial necrosis (leading to dissection) and ectopia of the pigment layer of the iris onto the anterior surface of the iris. In 1 patient this created an appearance suggesting coloboma. This anomaly was referred to as iris flocculi by Lewis and Merin (1995), who likewise found association with familial aortic dissection; see AAT6, 611788.
Erdheim cystic medial necrosis with dissecting aneurysm was reported in brothers (Graham and Milne, 1952; von Meyenburg, 1939), in father and son (Fleming and Helwig, 1941), and in mother and daughter (Griffiths et al., 1951), but clinical information in these reports was too scanty to permit exclusion of the Marfan syndrome (154700). Hanley and Jones (1967) reported dissecting aortic aneurysm in 2 sisters and the son of one of them. No stigmata of Marfan syndrome were present. Humphries et al. (1972) reported dissecting aortic aneurysm in mother and daughter. Lichtenstein (1972) reported Erdheim cystic medial necrosis in father and son. Opitz (1973) studied a family with isolated medial necrosis in a young woman, her father, and her father's father, and later (Opitz, 1982) referred to 2 other well-documented families.
McManus et al. (1986) described a family in which 6 members spanning 3 generations died of acute dissection of the aorta; 5 were men who died at a mean age of 28 years (range 22 to 34), while the sixth was the proband's paternal grandmother, who died at 62 years of age. All were hypertensive. Dilatation of the aortic root or aneurysms in the aorta were absent.
Toyama et al. (1989) observed acute aortic dissection in 3 of 4 sibs without signs of Marfan syndrome. The mother died at age 55 of acute dissection, as did several of her sibs.
Pyeritz (1990) pointed out that in the familial aortic dissection cases the aortic root often does not have the bulbous appearance characteristic of the Marfan syndrome. Furthermore, aortic dissection may occur with degrees of dilatation in the first part of the ascending aorta that would not ordinarily be considered dangerous in the Marfan syndrome.
Vaughan et al. (2001) reported 3 families in which thoracic aortic aneurysms associated, in some individuals, with aneurysms elsewhere in the arterial tree and segregated as an autosomal dominant trait. Marfan syndrome (154700) and Ehlers-Danlos syndrome (130050) had been excluded on phenotypic grounds. In family ANA the proband had presented at the age of 36 with a 5.7-cm ascending thoracic aortic aneurysm and a type I aortic dissection. Aortic pathology revealed cystic medial necrosis. Echocardiographic screening detected 13 additional affected family members from 20 at risk, with ages ranging from 2.5 to 65. Sinuses of Valsalva were dilated in all but 1 affected person. One individual had an abdominal aortic aneurysm and another had an aneurysm of the left subclavian artery. In family ANB the proband had repair of a type I aortic dissection at the age of 34 and subsequently underwent repair of abdominal and aortic arch aneurysms. Seven of 14 at-risk family members had dilated aortas. In the third family (ANF), the proband again presented in his early thirties with a thoracic aortic aneurysm. Seven of 11 at-risk family members had aortic dilatation at the level of the sinuses of Valsalva. One affected individual had bilateral fifth digit contractures, retinal detachment, and cataracts, and a further individual had similar finger contractures and mild pectus excavatum.
Loscalzo et al. (2007) performed a prospective study of 13 families with biscuspid aortic valve (BAV; 607086) and thoracic aortic aneurysm. All 13 families had multiple affected members, often in more than 1 generation, consistent with an autosomal dominant pattern of inheritance. Thirty-five percent (39/110) of family members had BAV/AAT or TAA alone. Two families had nonmanifesting obligate carriers, and 3 families had additional left outflow tract anomalies including coarctation of the aorta in 2 families and hypoplastic left heart syndrome in 1 family, suggesting reduced penetrance and variable expressivity. Loscalzo et al. (2007) suggested that BAV and AAT are independent manifestations of a single gene defect. They sequenced the TGFBR1 (190181) and TGFBR2 (190182) genes in 1 proband in each family, but found no mutations in either gene.
MappingAmong the families reported by Vaughan et al. (2001) with autosomal dominant thoracic aortic aneurysms, linkage to FBN1 (134797), FBN2 (612570), COL3A1 (120180), and the locus on 5q13-q14 (AAT2; 607087) was initially excluded in family ANA. An initial genomewide linkage analysis using highly polymorphic STRs revealed linkage by 2-point lod score to chromosome 11q microsatellites, with a maximum lod score of 3.35 at theta = 0.06 at marker D11S1356. Further multipoint analyses revealed a maximum lod score of 4.4 at a locus 1.1 cM telomeric to D11S1356, and haplotype analyses refined the disease interval to a 2.3-cM locus at chromosome 11q23.3-q24 between D11S1341 and AFMB031WC9. Candidate genes mapping to this interval were screened for mutations, but none was identified. The authors assigned the symbol FAA1 to this locus. Family ANB was linked to FBN1, and linkage to both FBN1 and FAA1 was excluded in family ANF.
Molecular GeneticsAssociations Pending Confirmation
For discussion of a possible association between variation in the MAT2A gene and thoracic aortic aneurysm, see 601468.0001.