Moebius Syndrome
Description
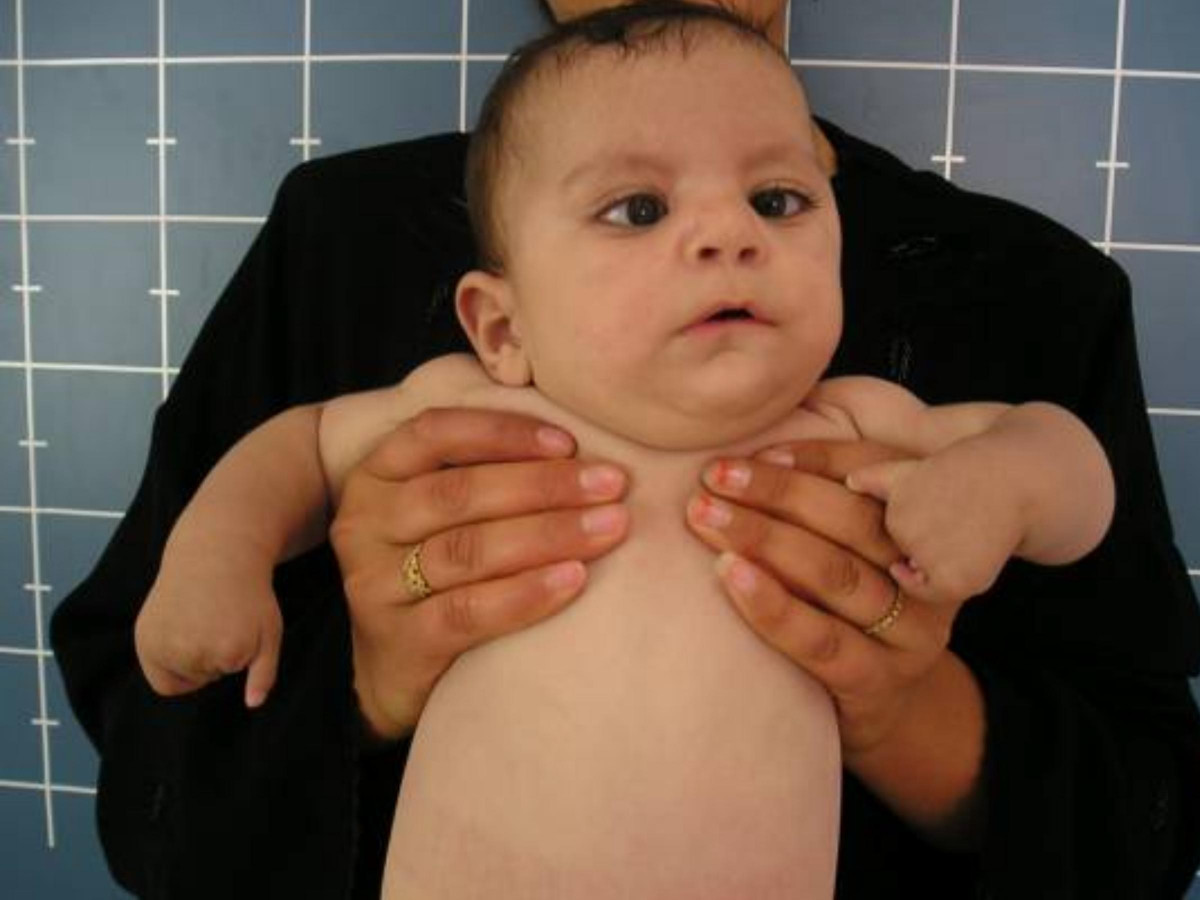
The most basic description of Moebius syndrome is a congenital facial palsy with impairment of ocular abduction. The facial nerve (cranial nerve VII) and abducens nerve (CN VI) are most frequently involved, but other cranial nerves may be involved as well. Other variable features include orofacial dysmorphism and limb malformations. Mental retardation has been reported in a subset of patients. Most cases of Moebius syndrome are sporadic, but familial occurrence has been reported (Verzijl et al., 2003).
The definition of and diagnostic criteria for Moebius syndrome have been controversial and problematic. The syndrome has most frequently been confused with hereditary congenital facial paresis (see 601471), which is restricted to involvement of the facial nerve and no other abnormalities. Verzijl et al. (2003) and Verzijl et al. (2005) concluded that HCFP and Moebius syndrome are distinct disorders, and that Moebius syndrome is a complex developmental disorder of the brainstem.
Moebius syndrome was defined at the Moebius Syndrome Foundation Research Conference in 2007 as congenital, nonprogressive facial weakness with limited abduction of one or both eyes. Additional features can include hearing loss and other cranial nerve dysfunction, as well as motor, orofacial, musculoskeletal, neurodevelopmental, and social problems (summary by Webb et al., 2012).
Kumar (1990) provided a review of Moebius syndrome, which was critiqued by Lipson et al. (1990). Briegel (2006) provided a review of Moebius sequence with special emphasis on neuropsychiatric findings.
Clinical FeaturesCongenital nonprogressive facial and abducens palsy was first described by Graefe (1880) and Moebius (1888). Harvey (1982) noted that Thomas had described the disorder in 1898. Congenital paralysis of the sixth and seventh cranial nerves was observed in multiple members of families by Wilbrand and Saenger (1921). Sprofkin and Hillman (1956) described a patient with arthrogryposis and Moebius syndrome who had a sib with arthrogryposis only.
Hanissian et al. (1970) reported the Moebius syndrome in black male twins. The facial nerves were small or absent at autopsy in both cases. Steigner et al. (1975) presented 6 cases of association of limb deficiencies with cranial nerve palsies. Some of these patients may have had isolated hereditary congenital facial paresis.
Wishnick et al. (1981) described a family in which 6 persons in 2 generations had sixth and/or seventh cranial nerve palsy with associated skeletal and/or digital anomalies. In 9 others, digital anomalies occurred apparently without cranial nerve involvement. EMG studies confirmed the involvement of the cranial nerves. Wishnick (1989) reported that she had no further information on this family and had seen no similar cases.
Stabile et al. (1984) presented a family with variable features of the Moebius syndrome in 3 members. The proband had complete VI and VII cranial nerve palsy and mental retardation. His brother had left convergent strabismus and mental retardation. His sister had only mental retardation. Brainstem auditory-evoked potentials (BAEP) were abnormal in the proband and sister, consistent with dysfunction at a supranuclear level. BAEPs were not tested in the third affected sib; they were normal in the mother. The authors suggested that the CNS is more generally involved than cranial nerves alone. Harbord et al. (1989) described a child with Moebius syndrome associated with unilateral cerebellar hypoplasia.
Verzijl et al. (2003) examined 37 Dutch patients with Moebius syndrome, defined as having, at a minimum, congenital facial weakness with impairment of ocular abduction. Ages ranged from 6 months to 53 years; 2 patients were related as mother and son. Nine patients were hypotonic at birth; 7 patients had neonatal respiratory difficulties, including 2 who died as neonates. Most patients (92%) had bilateral facial weakness and 62% of these patients had relative sparing of the lower half of the face. Twelve patients (34%) had Duane retraction anomaly and 3 patients had congenital fibrosis of the extraocular muscles resulting from developmental anomaly of the oculomotor or trochlear nuclei. Thirty-two patients (86%) had feeding problems at birth due to palatal and pharyngeal involvement. Other features included tongue hypoplasia (77%), nasal dysarthria (76%), and delayed language development (55%). Six patients (16%) had weak bite or absence of jaw rotation during chewing. Four patients had loss of sensation of the lip, cheek, forehead, and cornea, indicating a partial defect of the sensory root of the trigeminal nerve. Many patients had craniofacial abnormalities, including epicanthal folds (89%), flattened nasal bridge (81%), micrognathia (64%), high-arched palate (61%), external ear defects (47%), teeth defects (33%), and hypertelorism (25%). Two patients were autistic, 12 attended special education schools, and the rest had a normal education. Thirty-one (86%) patients had malformations of the extremities, including brachydactyly, clinodactyly, syndactyly, metacarpal abnormalities, pes planus, hypoplasia of the lower legs, talipes equinovarus, and arthrogryposis multiplex. Four patients (11%) had Poland syndrome. Thirty patients (88%) had motor disabilities with clumsy motor movements and poor coordination. Verzijl et al. (2003) concluded that consistent with maldevelopment of the rhombencephalon, involving both nuclei and corticospinal or corticobulbocerebellar long tracts.
Based on questionnaires filled out by 13 primary caregivers of preschool children with Moebius syndrome, Briegel et al. (2007) found that 16.7% had behavioral problems, a similar value to that of preschool children without the disorder. Boys had significantly higher scores than girls on aggressive behavior, oppositional defiance, and anxiety. Only 1 child was described as mentally retarded. Primary caregivers reported increased stress compared to controls.
Neuroradiologic Findings
In MRI studies of 3 patients with Moebius syndrome, 2 of whom were adults, Pedraza et al. (2000) found straightening of the floor of the fourth ventricle and absence of the medial colliculus at the level of the pons, suggesting hypoplasia of the VIth (abducens) and VIIth (facial) nuclei. There was also absence of the hypoglossal eminence at the medulla, consistent with hypoplasia of the hypoglossal nuclei. The findings correlated with the clinical findings.
Verzijl et al. (2005) reported MRI findings in 6 Dutch patients with Moebius syndrome ranging in age from 12 to 23 years. Cranial nerve VII (facial) was not identified bilaterally in 5 of the 6 patients; the last case was inconclusive, but suggestive of CN VII absence. The mean anteroposterior diameter of the pons in Moebius patients was less than that observed in 20 controls (19.7 mm and 22.5 mm, respectively). No tegmental calcifications were seen. Three patients had congenital anomalies of the posterior fossa, including Arnold-Chiari malformation, pineal cyst, and hypoplastic hemicerebellum and asymmetric lateral ventricles in 1. Verzijl et al. (2005) noted that hypoplasia of the brainstem had been described in 32% of the reported radiologic literature on Moebius syndrome. The authors concluded that the MRI findings were consistent with the theory that Moebius syndrome is a complex congenital developmental anomaly of the posterior fossa and rhombencephalon.
Associated Syndromes
Parker et al. (1981) reported at least 12 well-documented cases of association of Moebius and Poland (173800) syndromes. They concluded that the association represents a formal genesis malformation syndrome.
Kawai et al. (1990) reported a Japanese boy with Moebius syndrome associated with peripheral neuropathy and hypogonadotropic hypogonadism. Pulsatile administration of gonadotropin-releasing hormone for 3 months was mildly effective. Jennings et al. (2003) described a patient with Moebius syndrome associated with hypogonadotropic hypogonadism. They indicated that 5 other cases had previously been described by Olson et al. (1970), Rubinstein et al. (1975), Abid et al. (1978), Brackett et al. (1991), and Baraitser and Rudge (1996).
InheritanceAffected members of the family of Krueger and Friedrich (1963) occurred in 3 generations, consistent with autosomal dominant inheritance.
Legum et al. (1981) reported 3 unrelated families with Moebius syndrome. One family was consistent with autosomal dominant inheritance, another family was consanguineous and consistent with autosomal recessive inheritance, and the third was a sporadic case with no family history. All affected patients had paresis of the facial and abducens nerves, with variable involvement of the other cranial nerves. Baraitser (1977, 1982) stated that when myopathies have been excluded, the familial recurrence risk in the Moebius syndrome is no higher than 1 in 50. Baraitser (1982) suggested that some of the patients of Legum et al. (1981) had a congenital myopathy, as they demonstrated total external ophthalmoplegia.
Journel et al. (1989) described Moebius syndrome in 2 brothers and a male first cousin who were sons of sisters, thus suggesting X-linked recessive inheritance. The 2 brothers also had hypoplasia of the thumbs and absence of the big toes.
MacDermot et al. (1991) urged that the term 'Moebius syndrome' be restricted to cases with congenital sixth and seventh nerve paralysis with skeletal defects. They estimated a low familial recurrence risk, at about 2%. In their review, no recurrence was noted in 31 cases with cranial nerve palsies associated with oral abnormalities and limb defects. The features in an index case that may indicate a higher risk of recurrence are the absence of skeletal defects, isolated facial palsy, deafness, ophthalmoplegia, and digital contractures. As an example of the latter category, MacDermot et al. (1991) described mother and son with fifth, sixth, seventh, and bulbar cranial nerve paralysis who had 2 similarly affected relatives: the mother's maternal aunt and her son.
Graziadio et al. (2010) reported a mother and son with Moebius syndrome and skeletal anomalies. In infancy, the son was found to have a left facial palsy manifest as asymmetric crying facies, difficulty closing the eyes, and motility restriction of the left eye in all directions. He also had bilateral restriction of the hips and knees and clubfeet. Radiographic studies showed right coxa vara, left hip luxation, and osteopenia. At age 1 year, he had an expressionless face, sparse hair, high forehead, bitemporal narrowing, a broad but depressed nasal bridge, and strabismus. Other features included dimples in the elbows, short and tapering fingers, and sacral dimple. He had several surgeries to correct the lower limb skeletal anomalies. His 34-year-old mother had an expressionless face, strabismus, miotic pupils, bilateral facial palsy, osteopenia, hypoplasia of the iliac bones, coxa valga, small deformities of the left tibia and fibula, and clubfeet.
PathogenesisTowfighi et al. (1979) proposed 4 categories of Moebius syndrome based on neuropathologic findings in 15 cases: group I, characterized by hypoplasia of cranial nerve nuclei resulting from congenital rhombomeric maldevelopment; group II, characterized by neuronal loss and neuronal degeneration secondary to a defect in the facial peripheral nerve; group III, characterized by decreased neurons as well as degeneration, focal necrosis, gliosis, and calcifications in the brainstem nuclei due to vascular insufficiency or infection; and group IV, characterized by primary myopathic changes without lesions in the cranial nerve nuclei or nerves.
In a sporadic case of Moebius syndrome who died at age 11 days, Verzijl et al. (2005) found that the brainstem was asymmetric and hypoplastic with dysplastic, centrally located inferior olivary nuclei and dysplastic pyramidal tracts which failed to decussate. There was neuronal loss, gliosis, and microcalcifications in the brainstem affecting the medial reticular nuclei, and the VIth, VIIth, and XIIth cranial nerve nuclei. The findings were distinct from those seen in 3 cases of hereditary congenital facial palsy (see 601471), which showed isolated loss of facial nerve nuclei and no other abnormalities of the brainstem or posterior fossa. Verzijl et al. (2005) concluded that Moebius syndrome and HCFP are distinct disorders.
Verzijl et al. (2005) found a spectrum of electrophysiologic abnormalities in 11 patients with Moebius syndrome, suggesting defects at the supranuclear, nuclear, or peripheral levels in different patients. The authors concluded that Moebius syndrome is a complex regional developmental disorder of the brainstem.
Cattaneo et al. (2006) reported electrophysiologic studies of 24 unrelated individuals with sporadic Mobius syndrome that suggested 2 distinct pathogenic mechanisms. In the first group, 8 patients had increased distal motor latency after facial nerve stimulation, usually associated with decreased motor unit action potentials or polyphasic waveforms. In the second group, 9 patients with normal facial motor conduction showed increased motor unit action potentials indicative of a chronic neuropathic pattern and consistent with enlargement of the surviving motor units after axonal or motoneuronal loss. Cattaneo et al. (2006) hypothesized that the first group may have a genetically determined rhomboencephalic malformation disorder characterized by a population of small-sized motor neurons in the facial nucleus. In contrast, the second group may have a prenatally acquired axonal or motoneuronal loss. The functional impairment of facial muscles in both groups showed little evidence of brainstem interneuronal involvement and was most likely due to a nuclear or peripheral site of the lesion.
CytogeneticsZiter et al. (1977) observed congenital facial diplegia and flexion finger contractures in 7 members of 3 generations of a family. Each affected member showed an identical chromosome abnormality, reciprocal translocation between chromosomes 1p34 and 13q13. Unaffected family members had normal karyotypes. Slee et al. (1991) observed deletion of 13q12.2 in a 2.5-year-old girl with Moebius syndrome. Both observations suggested that a gene responsible for Moebius syndrome is located in region 13q12.2-q13.
Nishikawa et al. (1997) reported a boy with a Moebius-like syndrome associated with a 1;2 chromosome reciprocal translocation: t(1;2)(p22.3;q21.1). The patient had facial diplegia, ptosis, anteverted nostrils, malformed and low-set ears, and slight developmental delay. Donahue et al. (1993) had described Moebius syndrome with Poland syndrome, cleft palate, dextrocardia, mandibular hypoplasia, and multiple areas of diffuse brain volume loss in association with a reciprocal translocation between chromosomes 1 and 11: t(1;11)(p22;p13). Nishikawa et al. (1997) suggested that a disrupted gene at 1p22 may be the cause of the syndrome observed in their patient.
Population GeneticsVerzijl et al. (2003) estimated the incidence of Moebius syndrome in the Dutch population in 1996 to be 0.002% of births (4 in 189,000 births).
Molecular GeneticsAssociations Pending Confirmation
For a possible association between Moebius syndrome and de novo heterozygous variants in the REV3L and PLXND1 genes, see 602776 and 604282, respectively.
HistorySteinberg (2005) provided a history of Paul Julius Moebius, a German neurologist who lived from 1853 to 1907.