Buschke-Ollendorff Syndrome
A number sign (#) is used with this entry because of evidence that Buschke-Ollendorff syndrome (BOS) is caused by heterozygous loss-of-function mutations in the LEMD3 gene (607844) on chromosome 12q14.
DescriptionBuschke-Ollendorff syndrome is an autosomal dominant connective tissue disorder manifest by multiple subcutaneous nevi or nodules. They may be either elastin-rich (elastoma) or collagen-rich (dermatofibrosis lenticularis disseminata) on histologic examination. The lesions are usually nontender and firm. Affected individuals also have osteopoikilosis (OPK), literally meaning 'spotted bones,' which are osteosclerotic foci that occur in the epiphyses and metaphyses of long bones, wrist, foot, ankle, pelvis, and scapula. Some individuals have both skin and bone manifestations, whereas others may lack skin or bone manifestations. Some individuals may also have melorheostosis (155950), which is characterized by 'flowing' hyperostosis of the cortex of tubular bones. Most reported cases of BOS and OPK are benign, and the bone lesions are found incidentally, although some patients may have joint pain (reviews by Hellemans et al., 2004 and Zhang et al., 2009).
Clinical FeaturesBerlin et al. (1967) showed that either the skin or the bone lesions of osteopoikilosis can be absent in families in which some members have both.
Verbov (1977) described disseminated dermatofibrosis associated with osteopoikilosis in a 47-year-old woman whose 2 children also had typical skin lesions said to have been present from birth. The proposita was referred because of the new development of lumps over the lower back and buttocks. She had had an asymptomatic, small, smooth, whitish-yellow, scar-like plaque over the right anterior thigh for many years. Her mother had had a similar lump over the left thigh from childhood. Biopsy of a lump showed dense masses of collagen. A yellow patch on the right buttock showed increase in elastic fibers which were clumped.
Walpole and Manners (1990) reported the cases of a mother and 2 daughters. The characteristic dermal connective tissue nevi become less obvious with time. The spotty lesions of sclerosis showed melorheostosis (155950), e.g., in the upper humerus. Connective tissue nevi involved buttocks, proximal limbs, and trunk, and were present early in life and asymmetrical in distribution. Muscle fibrosis and contractures were present in one of the cases and have been reported by others.
Giro et al. (1992) described Buschke-Ollendorff syndrome in multiple members of 4 or more generations of 2 families in an autosomal dominant pedigree pattern. Elastin mRNA levels were elevated in all cultured fibroblasts from patients. The proband of 1 family, a 58-year-old man, had been found to have osteopoikilosis at the age of 28 when he was evaluated for stiff joints. He and other members of his family had skin nodules accompanied by prominent joint stiffness, especially affecting the ankles and shoulders. He was described as having thousands of 1- to 2-mm yellow papules prominent on volar wrists, inner arms, and legs, sparing the face and neck. There were firm, 1- to 2-cm fibrous nodules on the trunk. Joint mobility was limited at the ankles and shoulders. Four- to 5-mm flesh-colored papules were present on the tongue. The patient was hoarse; laryngoscopy showed leukoplakia.
Sarralde et al. (1994) described osteopoikilosis in a 42-year-old woman and in 3 of her 6 children. Typical radiologic features were the only finding; none had skin lesions.
Woodrow et al. (2001) reported a family with Buschke-Ollendorff syndrome presenting as elastic tissue nevi without evidence of osteopoikilosis. They discussed the variable expression of this syndrome in other families reported in the literature, the association of various connective tissue abnormalities, and their correlation with the pathogenesis of this condition. The connective tissue nevi most commonly found are juvenile elastomas. There is ultrastructural evidence for both collagen and elastin being abnormal in the lesional skin of patients with BOS. Elastin fibers are broad, interlacing, and lack supporting microfibrils, while collagen fibers are variable in diameter and show bizarre morphologic forms, including collagen flowers (Uitto et al., 1981). There are also pathognomonic changes in elastin synthesis from lesional fibroblasts: lesional fibroblasts produce 2 to 8 times more tropoelastin (elastin precursor) compared with control fibroblasts (Morrison et al., 1977) and there is elevation of steady-state elastin mRNA levels possibly secondary to the stimulation of elastin gene transcription or stabilization of elastin mRNA levels (Davidson et al., 1995).
Hellemans et al. (2004) summarized clinical features of osteopoikilosis, Buschke-Ollendorff syndrome, and the related disorder melorheostosis (155950). Osteopoikilosis is an autosomal dominant skeletal dysplasia characterized by a symmetric but unequal distribution of multiple hyperostotic areas in different parts of the skeleton. These lesions, usually detected incidentally, represent foci of old remodeled bone with lamellar structure, either connected to adjacent trabeculae of spongy bone or attached to the subchondral cortex. Osteopoikilosis can occur either as an isolated anomaly or in association with other abnormalities of skin and bone. Buschke-Ollendorff syndrome, an autosomal dominant disorder, refers to the association of osteopoikilosis with disseminated connective tissue nevi. Both elastic-type nevi (juvenile elastoma) and collagen-type nevi (dermatofibrosis lenticularis disseminata) have been described in Buschke-Ollendorff syndrome. Skin or bony lesions can be absent in some family members, whereas other relatives have both. The cooccurrence of osteopoikilosis and melorheostosis has been observed in a few instances. Melorheostosis is characterized by a 'flowing' (rheos) hyperostosis of the cortex of tubular bones. These lesions are usually asymmetric: they may involve only 1 limb or correspond to a particular sclerotome. They are often accompanied by abnormalities of adjacent soft tissues, such as joint contractures, sclerodermatous skin lesions, muscle atrophy, hemangiomas, and lymphedema.
Clinical Variability
Butkus et al. (1997) reported a family in which 4 sibs had generalized osteopoikilosis. One was a 40-year-old woman who also had melorheostosis (155950), which was not found in the other sibs. Butkus et al. (1997) suggested that a somatic mutation at the same locus may be responsible for the melorheostosis. Nevin et al. (1999) reported a family similar to that of Butkus et al. (1997): a 19-year-old woman with melorheostosis and osteopoikilosis whose mother and sister had osteopoikilosis without evidence of melorheostosis. Debeer et al. (2003) described a 3-generation family with clinical and radiologic findings of osteopoikilosis in 5 members and melorheostosis in 1 individual. Debeer et al. (2003) noted that the findings in this family strengthened the hypothesis that osteopoikilosis is an autosomal dominant condition and that an early postzygotic second hit mutation in the second allele could result in melorheostosis. Butkus et al. (1997), Nevin et al. (1999), and Debeer et al. (2003) all suggested that the melorheostotic component was due to a second mutation at the same locus as that causing familial osteopoikilosis, a well-established autosomal dominant trait.
However, in the family reported by Debeer et al. (2003), Hellemans et al. (2004) found a germline loss-of-function mutation in the LEMD3 gene (607844.0004). A somatic mutation in the second LEMD3 allele was not found in skin fibroblasts from the patient with melorheostosis. The authors concluded that the failure to find such a somatic mutation suggests that other genetic factors contribute to the presence and distribution of skin and bone lesions in these disorders. In addition, Mumm et al. (2007) identified a heterozygous nonsense mutation (R655X; 607844.0008) in the family reported by Butkus et al. (1997).
Hershkovitz et al. (2007) reported a 2-generation family of Jewish origin in which 2 first cousins, ages 6 and 7 years, respectively, had asymptomatic flesh-colored cutaneous nodules localized mainly over the extremities and lower trunk. Neither child had any other manifestations. The authors referred to this disorder as 'familial cutaneous collagenoma' (115250), but noted the phenotypic overlap with Buschke-Ollendorff syndrome. Neither child had evidence of bone lesions, as found in BOS, but individuals with BOS may not show bone lesions. A heterozygous mutation in the LEMD3 gene (607844.0009) was found in both children, but it was also found in the unaffected father of 1 of the children. Hershkovitz et al. (2007) suggested that the findings indicated that abnormal function of LEMD3 may may be causally associated with familial collagenomas, and that this family had a variant of BOS without bony abnormalities.
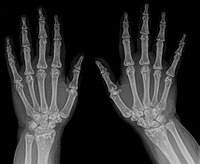
Zhang et al. (2009) reported a 9-year-old Italian boy and his father with Buschke-Ollendorff syndrome. The boy developed progressive coalescing flesh-colored subcutaneous nodules on his left thigh and right arm over several years. They were nontender and firm. Histologic studies showed connective tissue nevi of the elastic type. He also had mild signs of osteopoikilosis demonstrated by radiographs showing hyperostotic lesions on the proximal and distal humeral epiphyses. The father had no definitive signs of connective tissue nevus but had classic widespread osteopoikilosis of the knees, hands, wrists, and pelvis. Genetic analysis identified a heterozygous nonsense mutation in the LEMD3 gene (W855X; 607844.0007). The findings illustrated intrafamilial variability in this disorder.
InheritanceStriking pedigrees supporting autosomal dominant inheritance were published by Melnick (1959), Jonasch (1955), and Busch (1937), among others. Landberg and Akesson (1963) observed the bone lesions in father and son. Raque and Wood (1970) found dermatoosteopoikilosis in a brother and sister and in a son of the brother.
Chigira et al. (1991) presented evidence that osteopoikilosis is an autosomal dominant trait.
MappingHellemans et al. (2004) demonstrated linkage of osteopoikilosis to 12q13, or specifically to a candidate region of 23.55 cM on 12q12-q14.3. They studied a patient with proportionate short stature, microcephaly, learning disabilities, ectopic kidneys, and osteopoikilosis. They hypothesized that this individual might have a microdeletion, resulting in the loss of several contiguous genes, including the gene mutated in osteopoikilosis. They indeed found a deletion in the area identified by the linkage study. Hellemans et al. (2004) then tested more markers in the region of overlap between the microdeletion and the linkage interval, allowing them to narrow the linkage interval and define a 3.07-Mb critical region for association with osteopoikilosis.
Molecular GeneticsHellemans et al. (2004) identified 23 known genes within the candidate region for osteopoikilosis that they had identified on chromosome 12 and searched for mutations in 2 candidate genes, WIF1 (605186) and LEMD3 (607844). No abnormalities were found in WIF1. Sequencing of LEMD3 identified loss-of-function mutations (see, e.g., 607844.0001-607844.0006) in all affected individuals in the 3 families studied and in 3 unrelated individuals with osteopoikilosis.
Nevin et al. (1999) and Happle (2004) suggested that the asymmetric distribution of skin lesions in Buschke-Ollendorff syndrome and the segmental involvement usually observed in melorheostosis (155950) result from a somatic mutation ('second hit'). To investigate this possibility, Hellemans et al. (2004) took skin biopsy samples from 2 affected individuals, one from an elastic-type nevus in a person with BOS and the second from a hard scleroderma-like lesion in an individual with melorheostosis. They found no evidence of a second hit in these 2 familial cases. The possibility that a somatic mutation in osteoblasts could explain the spotty occurrence of bone lesions could not be investigated because of unavailability of bone specimens.