Pancreatic Cancer

Pancreatic cancer arises when cells in the pancreas, a glandular organ behind the stomach, begin to multiply out of control and form a mass. These cancerous cells have the ability to invade other parts of the body. A number of types of pancreatic cancer are known.
The most common, pancreatic adenocarcinoma, accounts for about 90% of cases, and the term "pancreatic cancer" is sometimes used to refer only to that type. These adenocarcinomas start within the part of the pancreas that makes digestive enzymes. Several other types of cancer, which collectively represent the majority of the nonadenocarcinomas, can also arise from these cells. About 1–2% of cases of pancreatic cancer are neuroendocrine tumors, which arise from the hormone-producing cells of the pancreas. These are generally less aggressive than pancreatic adenocarcinoma.
Signs and symptoms of the most-common form of pancreatic cancer may include yellow skin, abdominal or back pain, unexplained weight loss, light-colored stools, dark urine, and loss of appetite. Usually, no symptoms are seen in the disease's early stages, and symptoms that are specific enough to suggest pancreatic cancer typically do not develop until the disease has reached an advanced stage. By the time of diagnosis, pancreatic cancer has often spread to other parts of the body.
Pancreatic cancer rarely occurs before the age of 40, and more than half of cases of pancreatic adenocarcinoma occur in those over 70. Risk factors for pancreatic cancer include tobacco smoking, obesity, diabetes, and certain rare genetic conditions. About 25% of cases are linked to smoking, and 5–10% are linked to inherited genes. Pancreatic cancer is usually diagnosed by a combination of medical imaging techniques such as ultrasound or computed tomography, blood tests, and examination of tissue samples (biopsy). The disease is divided into stages, from early (stage I) to late (stage IV). Screening the general population has not been found to be effective.
The risk of developing pancreatic cancer is lower among nonsmokers, and people who maintain a healthy weight and limit their consumption of red or processed meat. Smokers' chances of developing the disease decrease if they stop smoking and almost return to that of the rest of the population after 20 years. Pancreatic cancer can be treated with surgery, radiotherapy, chemotherapy, palliative care, or a combination of these. Treatment options are partly based on the cancer stage. Surgery is the only treatment that can cure pancreatic adenocarcinoma, and may also be done to improve quality of life without the potential for cure. Pain management and medications to improve digestion are sometimes needed. Early palliative care is recommended even for those receiving treatment that aims for a cure.
In 2015, pancreatic cancers of all types resulted in 411,600 deaths globally. Pancreatic cancer is the fifth most-common cause of death from cancer in the United Kingdom, and the third most-common in the United States. The disease occurs most often in the developed world, where about 70% of the new cases in 2012 originated. Pancreatic adenocarcinoma typically has a very poor prognosis; after diagnosis, 25% of people survive one year and 5% live for five years. For cancers diagnosed early, the five-year survival rate rises to about 20%. Neuroendocrine cancers have better outcomes; at five years from diagnosis, 65% of those diagnosed are living, though survival varies considerably depending on the type of tumor.
Types

The many types of pancreatic cancer can be divided into two general groups. The vast majority of cases (about 95%) occur in the part of the pancreas that produces digestive enzymes, known as the exocrine component. Several subtypes of exocrine pancreatic cancers are described, but their diagnosis and treatment have much in common. The small minority of cancers that arise in the hormone-producing (endocrine) tissue of the pancreas have different clinical characteristics and are called pancreatic neuroendocrine tumors, sometimes abbreviated as "PanNETs". Both groups occur mainly (but not exclusively) in people over 40, and are slightly more common in men, but some rare subtypes mainly occur in women or children.
Exocrine cancers
The exocrine group is dominated by pancreatic adenocarcinoma (variations of this name may add "invasive" and "ductal"), which is by far the most common type, representing about 85% of all pancreatic cancers. Nearly all these start in the ducts of the pancreas, as pancreatic ductal adenocarcinoma (PDAC). This is despite the fact that the tissue from which it arises – the pancreatic ductal epithelium – represents less than 10% of the pancreas by cell volume, because it constitutes only the ducts (an extensive but capillary-like duct-system fanning out) within the pancreas. This cancer originates in the ducts that carry secretions (such as enzymes and bicarbonate) away from the pancreas. About 60–70% of adenocarcinomas occur in the head of the pancreas.
The next-most common type, acinar cell carcinoma of the pancreas, arises in the clusters of cells that produce these enzymes, and represents 5% of exocrine pancreas cancers. Like the 'functioning' endocrine cancers described below, acinar cell carcinomas may cause over-production of certain molecules, in this case digestive enzymes, which may cause symptoms such as skin rashes and joint pain.
Cystadenocarcinomas account for 1% of pancreatic cancers, and they have a better prognosis than the other exocrine types.
Pancreatoblastoma is a rare form, mostly occurring in childhood, and with a relatively good prognosis. Other exocrine cancers include adenosquamous carcinomas, signet ring cell carcinomas, hepatoid carcinomas, colloid carcinomas, undifferentiated carcinomas, and undifferentiated carcinomas with osteoclast-like giant cells. Solid pseudopapillary tumor is a rare low-grade neoplasm that mainly affects younger women, and generally has a very good prognosis.
Pancreatic mucinous cystic neoplasms are a broad group of pancreas tumors that have varying malignant potential. They are being detected at a greatly increased rate as CT scans become more powerful and common, and discussion continues as how best to assess and treat them, given that many are benign.
Neuroendocrine
The small minority of tumors that arise elsewhere in the pancreas are mainly pancreatic neuroendocrine tumors (PanNETs). Neuroendocrine tumors (NETs) are a diverse group of benign or malignant tumors that arise from the body's neuroendocrine cells, which are responsible for integrating the nervous and endocrine systems. NETs can start in most organs of the body, including the pancreas, where the various malignant types are all considered to be rare. PanNETs are grouped into 'functioning' and 'nonfunctioning' types, depending on the degree to which they produce hormones. The functioning types secrete hormones such as insulin, gastrin, and glucagon into the bloodstream, often in large quantities, giving rise to serious symptoms such as low blood sugar, but also favoring relatively early detection. The most common functioning PanNETs are insulinomas and gastrinomas, named after the hormones they secrete. The nonfunctioning types do not secrete hormones in a sufficient quantity to give rise to overt clinical symptoms, so nonfunctioning PanNETs are often diagnosed only after the cancer has spread to other parts of the body.
As with other neuroendocrine tumors, the history of the terminology and classification of PanNETs is complex. PanNETs are sometimes called "islet cell cancers", though they are now known to not actually arise from islet cells as previously thought.
Signs and symptoms

Since pancreatic cancer usually does not cause recognizable symptoms in its early stages, the disease is typically not diagnosed until it has spread beyond the pancreas itself. This is one of the main reasons for the generally poor survival rates. Exceptions to this are the functioning PanNETs, where over-production of various active hormones can give rise to symptoms (which depend on the type of hormone).
Bearing in mind that the disease is rarely diagnosed before the age of 40, common symptoms of pancreatic adenocarcinoma occurring before diagnosis include:
- Pain in the upper abdomen or back, often spreading from around the stomach to the back. The location of the pain can indicate the part of the pancreas where a tumor is located. The pain may be worse at night and may increase over time to become severe and unremitting. It may be slightly relieved by bending forward. In the UK, about half of new cases of pancreatic cancer are diagnosed following a visit to a hospital emergency department for pain or jaundice. In up to two-thirds of people, abdominal pain is the main symptom, for 46% of the total accompanied by jaundice, with 13% having jaundice without pain.
- Jaundice, a yellow tint to the whites of the eyes or skin, with or without pain, and possibly in combination with darkened urine, results when a cancer in the head of the pancreas obstructs the common bile duct as it runs through the pancreas.
- Unexplained weight loss, either from loss of appetite, or loss of exocrine function resulting in poor digestion.
- The tumor may compress neighboring organs, disrupting digestive processes and making it difficult for the stomach to empty, which may cause nausea and a feeling of fullness. The undigested fat leads to foul-smelling, fatty feces that are difficult to flush away. Constipation is also common.
- At least 50% of people with pancreatic adenocarcinoma have diabetes at the time of diagnosis. While long-standing diabetes is a known risk factor for pancreatic cancer (see Risk factors), the cancer can itself cause diabetes, in which case recent onset of diabetes could be considered an early sign of the disease. People over 50 who develop diabetes have eight times the usual risk of developing pancreatic adenocarcinoma within three years, after which the relative risk declines.
Other findings
- Trousseau's syndrome, in which blood clots form spontaneously in the portal blood vessels, the deep veins of the extremities, or the superficial veins anywhere on the body, may be associated with pancreatic cancer, and is found in about 10% of cases.
- Clinical depression has been reported in association with pancreatic cancer in some 10–20% of cases, and can be a hindrance to optimal management. The depression sometimes appears before the diagnosis of cancer, suggesting that it may be brought on by the biology of the disease.
Other common manifestations of the disease include weakness and tiring easily, dry mouth, sleep problems, and a palpable abdominal mass.
Symptoms of spread

The spread of pancreatic cancer to other organs (metastasis) may also cause symptoms. Typically, pancreatic adenocarcinoma first spreads to nearby lymph nodes, and later to the liver or to the peritoneal cavity, large intestine, or lungs. Uncommonly, it spreads to the bones or brain.
Cancers in the pancreas may also be secondary cancers that have spread from other parts of the body. This is uncommon, found in only about 2% of cases of pancreatic cancer. Kidney cancer is by far the most common cancer to spread to the pancreas, followed by colorectal cancer, and then cancers of the skin, breast, and lung. Surgery may be performed on the pancreas in such cases, whether in hope of a cure or to alleviate symptoms.
Risk factors
Risk factors for pancreatic adenocarcinoma include:
- Age, sex, and ethnicity – the risk of developing pancreatic cancer increases with age. Most cases occur after age 65, while cases before age 40 are uncommon. The disease is slightly more common in men than in women. In the United States, it is over 1.5 times more common in African Americans, though incidence in Africa is low.
- Cigarette smoking is the best-established avoidable risk factor for pancreatic cancer, approximately doubling risk among long-term smokers, the risk increasing with the number of cigarettes smoked and the years of smoking. The risk declines slowly after smoking cessation, taking some 20 years to return to almost that of nonsmokers.
- Obesity - a body mass index greater than 35 increases relative risk by about half.
- Family history – 5–10% of pancreatic cancer cases have an inherited component, where people have a family history of pancreatic cancer. The risk escalates greatly if more than one first-degree relative had the disease, and more modestly if they developed it before the age of 50. Most of the genes involved have not been identified. Hereditary pancreatitis gives a greatly increased lifetime risk of pancreatic cancer of 30–40% to the age of 70. Screening for early pancreatic cancer may be offered to individuals with hereditary pancreatitis on a research basis. Some people may choose to have their pancreas surgically removed to prevent cancer from developing in the future.
- Pancreatic cancer has been associated with these other rare hereditary syndromes: Peutz–Jeghers syndrome due to mutations in the STK11 tumor suppressor gene (very rare, but a very strong risk factor); dysplastic nevus syndrome (or familial atypical multiple mole and melanoma syndrome, FAMMM-PC) due to mutations in the CDKN2A tumor suppressor gene; autosomal recessive ataxia-telangiectasia and autosomal dominantly inherited mutations in the BRCA2 and PALB2 genes; hereditary non-polyposis colon cancer (Lynch syndrome); and familial adenomatous polyposis. PanNETs have been associated with multiple endocrine neoplasia type 1 (MEN1) and von Hippel Lindau syndromes.
- Chronic pancreatitis appears to almost triple risk, and as with diabetes, new-onset pancreatitis may be a symptom of a tumor. The risk of pancreatic cancer in individuals with familial pancreatitis is particularly high.
- Diabetes mellitus is a risk factor for pancreatic cancer and (as noted in the Signs and symptoms section) new-onset diabetes may also be an early sign of the disease. People who have been diagnosed with type 2 diabetes for longer than 10 years may have a 50% increased risk, as compared with individuals without diabetes.
- Specific types of food (as distinct from obesity) have not been clearly shown to increase the risk of pancreatic cancer. Dietary factors for which some evidence shows slightly increased risk include processed meat, red meat, and meat cooked at very high temperatures (e.g. by frying, broiling, or grilling).
Alcohol
Drinking alcohol excessively is a major cause of chronic pancreatitis, which in turn predisposes to pancreatic cancer, but considerable research has failed to firmly establish alcohol consumption as a direct risk factor for pancreatic cancer. Overall, the association is consistently weak and the majority of studies have found no association, with smoking a strong confounding factor. The evidence is stronger for a link with heavy drinking, of at least six drinks per day.
Pathophysiology

Precancer

Exocrine cancers are thought to arise from several types of precancerous lesions within the pancreas, but these lesions do not always progress to cancer, and the increased numbers detected as a byproduct of the increasing use of CT scans for other reasons are not all treated. Apart from pancreatic serous cystadenomas, which are almost always benign, four types of precancerous lesion are recognized.
The first is pancreatic intraepithelial neoplasia. These lesions are microscopic abnormalities in the pancreas and are often found in autopsies of people with no diagnosed cancer. These lesions may progress from low to high grade and then to a tumor. More than 90% of cases at all grades carry a faulty KRAS gene, while in grades 2 and 3, damage to three further genes – CDKN2A (p16), p53, and SMAD4 – are increasingly often found.
A second type is the intraductal papillary mucinous neoplasm (IPMN). These are macroscopic lesions, which are found in about 2% of all adults. This rate rises to about 10% by age 70. These lesions have about a 25% risk of developing into invasive cancer. They may have KRAS gene mutations (40–65% of cases) and in the GNAS Gs alpha subunit and RNF43, affecting the Wnt signaling pathway. Even if removed surgically, a considerably increased risk remains of pancreatic cancer developing subsequently.
The third type, pancreatic mucinous cystic neoplasm (MCN), mainly occurs in women, and may remain benign or progress to cancer. If these lesions become large, cause symptoms, or have suspicious features, they can usually be successfully removed by surgery.
A fourth type of cancer that arises in the pancreas is the intraductal tubulopapillary neoplasm. This type was recognised by the WHO in 2010 and constitutes about 1–3% of all pancreatic neoplasms. Mean age at diagnosis is 61 years (range 35–78 years). About 50% of these lesions become invasive. Diagnosis depends on histology, as these lesions are very difficult to differentiate from other lesions on either clinical or radiological grounds.
Invasive cancer
The genetic events found in ductal adenocarcinoma have been well characterized, and complete exome sequencing has been done for the common types of tumor. Four genes have each been found to be mutated in the majority of adenocarcinomas: KRAS (in 95% of cases), CDKN2A (also in 95%), TP53 (75%), and SMAD4 (55%). The last of these is especially associated with a poor prognosis. SWI/SNF mutations/deletions occur in about 10–15% of the adenocarcinomas. The genetic alterations in several other types of pancreatic cancer and precancerous lesions have also been researched. Transcriptomics analyses and mRNA sequencing for the common forms of pancreatic cancer have found that 75% of human genes are expressed in the tumors, with some 200 genes more specifically expressed in pancreatic cancer as compared to other tumor types.
PanNETs
The genes often found mutated in PanNETs are different from those in exocrine pancreatic cancer. For example, KRAS mutation is normally absent. Instead, hereditary MEN1 gene mutations give rise to MEN1 syndrome, in which primary tumors occur in two or more endocrine glands. About 40–70% of people born with a MEN1 mutation eventually develop a PanNet. Other genes that are frequently mutated include DAXX, mTOR, and ATRX.
Diagnosis
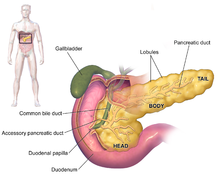


The symptoms of pancreatic adenocarcinoma do not usually appear in the disease's early stages, and they are not individually distinctive to the disease. The symptoms at diagnosis vary according to the location of the cancer in the pancreas, which anatomists divide (from left to right on most diagrams) into the thick head, the neck, and the tapering body, ending in the tail.
Regardless of a tumor's location, the most common symptom is unexplained weight loss, which may be considerable. A large minority (between 35% and 47%) of people diagnosed with the disease will have had nausea, vomiting, or a feeling of weakness. Tumors in the head of the pancreas typically also cause jaundice, pain, loss of appetite, dark urine, and light-colored stools. Tumors in the body and tail typically also cause pain.
People sometimes have recent onset of atypical type 2 diabetes that is difficult to control, a history of recent but unexplained blood vessel inflammation caused by blood clots (thrombophlebitis) known as Trousseau sign, or a previous attack of pancreatitis. A doctor may suspect pancreatic cancer when the onset of diabetes in someone over 50 years old is accompanied by typical symptoms such as unexplained weight loss, persistent abdominal or back pain, indigestion, vomiting, or fatty feces. Jaundice accompanied by a painlessly swollen gallbladder (known as Courvoisier's sign) may also raise suspicion, and can help differentiate pancreatic cancer from gallstones.
Medical imaging techniques, such as computed tomography (CT scan) and endoscopic ultrasound (EUS) are used both to confirm the diagnosis and to help decide whether the tumor can be surgically removed (its "resectability"). On contrast CT scan, pancreatic cancer typically shows a gradually increasing radiocontrast uptake, rather than a fast washout as seen in a normal pancreas or a delayed washout as seen in chronic pancreatitis. Magnetic resonance imaging and positron emission tomography may also be used, and magnetic resonance cholangiopancreatography may be useful in some cases. Abdominal ultrasound is less sensitive and will miss small tumors, but can identify cancers that have spread to the liver and build-up of fluid in the peritoneal cavity (ascites). It may be used for a quick and cheap first examination before other techniques.

A biopsy by fine needle aspiration, often guided by endoscopic ultrasound, may be used where there is uncertainty over the diagnosis, but a histologic diagnosis is not usually required for removal of the tumor by surgery to go ahead.
Liver function tests can show a combination of results indicative of bile duct obstruction (raised conjugated bilirubin, γ-glutamyl transpeptidase and alkaline phosphatase levels). CA19-9 (carbohydrate antigen 19.9) is a tumor marker that is frequently elevated in pancreatic cancer. However, it lacks sensitivity and specificity, not least because 5% of people lack the Lewis (a) antigen and cannot produce CA19-9. It has a sensitivity of 80% and specificity of 73% in detecting pancreatic adenocarcinoma, and is used for following known cases rather than diagnosis.
Histopathology
The most common form of pancreatic cancer (adenocarcinoma) is typically characterized by moderately to poorly differentiated glandular structures on microscopic examination. There is typically considerable desmoplasia or formation of a dense fibrous stroma or structural tissue consisting of a range of cell types (including myofibroblasts, macrophages, lymphocytes and mast cells) and deposited material (such as type I collagen and hyaluronic acid). This creates a tumor microenvironment that is short of blood vessels (hypovascular) and so of oxygen (tumor hypoxia). It is thought that this prevents many chemotherapy drugs from reaching the tumor, as one factor making the cancer especially hard to treat.
| Cancer type | Relative incidence | Microscopy findings | Micrograph | Immunohistochemistry markers | Genetic alterations |
|---|---|---|---|---|---|
| Pancreatic ductal adenocarcinoma (PDAC) | 90% | Glands and desmoplasia | 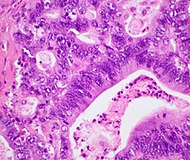 |
|
|
| Pancreatic acinar cell carcinoma (ACC) | 1% to 2% | Granular appearance | 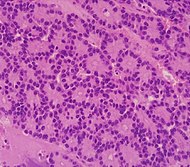
|
|
|
| Adenosquamous carcinoma | 1% to 4% | Combination of gland-like cells and squamous epithelial cells. |  |
Positive for:
Negative for:
|
|
| Pancreatic neuroendocrine tumor | 5% | Multiple nests of tumor cells | 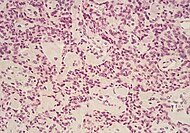 |
|
|
| Pre-cancer below for comparison: | |||||
| Precancer: Intraductal papillary mucinous neoplasm (IPMN) |
3% | Mucinous epithelial cells. Growth within the pancreatic ducts. | 
|
|
|
Staging
Exocrine cancers
Pancreatic cancer is usually staged following a CT scan. The most widely used cancer staging system for pancreatic cancer is the one formulated by the American Joint Committee on Cancer (AJCC) together with the Union for International Cancer Control (UICC). The AJCC-UICC staging system designates four main overall stages, ranging from early to advanced disease, based on TNM classification of Tumor size, spread to lymph Nodes, and Metastasis.
To help decide treatment, the tumors are also divided into three broader categories based on whether surgical removal seems possible: in this way, tumors are judged to be "resectable", "borderline resectable", or "unresectable". When the disease is still in an early stage (AJCC-UICC stages I and II), without spread to large blood vessels or distant organs such as the liver or lungs, surgical resection of the tumor can normally be performed, if the patient is willing to undergo this major operation and is thought to be sufficiently fit.
The AJCC-UICC staging system allows distinction between stage III tumors that are judged to be "borderline resectable" (where surgery is technically feasible because the celiac axis and superior mesenteric artery are still free) and those that are "unresectable" (due to more locally advanced disease); in terms of the more detailed TNM classification, these two groups correspond to T3 and T4 respectively.
- Pancreatic cancer staging (TNM classification)

Stage T1 pancreatic cancer

Stage T2 pancreatic cancer
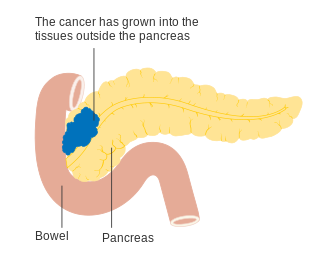
Stage T3 pancreatic cancer
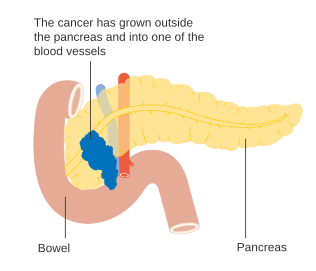
Stage T4 pancreatic cancer

Pancreatic cancer in nearby lymph nodes – Stage N1

Locally advanced adenocarcinomas have spread into neighboring organs, which may be any of the following (in roughly decreasing order of frequency): the duodenum, stomach, transverse colon, spleen, adrenal gland, or kidney. Very often they also spread to the important blood or lymphatic vessels and nerves that run close to the pancreas, making surgery far more difficult. Typical sites for metastatic spread (stage IV disease) are the liver, peritoneal cavity and lungs, all of which occur in 50% or more of fully advanced cases.
PanNETs
The 2010 WHO classification of tumors of the digestive system grades all the pancreatic neuroendocrine tumors (PanNETs) into three categories, based on their degree of cellular differentiation (from "NET G1" through to the poorly differentiated "NET G3"). The U.S. National Comprehensive Cancer Network recommends use of the same AJCC-UICC staging system as pancreatic adenocarcinoma.:52 Using this scheme, the stage-by-stage outcomes for PanNETs are dissimilar to those of the exocrine cancers. A different TNM system for PanNETs has been proposed by the European Neuroendocrine Tumor Society.
Prevention and screening
Apart from not smoking, the American Cancer Society recommends keeping a healthy weight, and increasing consumption of fruits, vegetables, and whole grains, while decreasing consumption of red and processed meat, although there is no consistent evidence this will prevent or reduce pancreatic cancer specifically. A 2014 review of research concluded that there was evidence that consumption of citrus fruits and curcumin reduced risk of pancreatic cancer, while there was possibly a beneficial effect from whole grains, folate, selenium, and non-fried fish.
In the general population, screening of large groups is not considered effective and may be harmful as of 2019, although newer techniques, and the screening of tightly targeted groups, are being evaluated. Nevertheless, regular screening with endoscopic ultrasound and MRI/CT imaging is recommended for those at high risk from inherited genetics.
Management
Exocrine cancer
A key assessment that is made after diagnosis is whether surgical removal of the tumor is possible (see Staging), as this is the only cure for this cancer. Whether or not surgical resection can be offered depends on how much the cancer has spread. The exact location of the tumor is also a significant factor, and CT can show how it relates to the major blood vessels passing close to the pancreas. The general health of the person must also be assessed, though age in itself is not an obstacle to surgery.
Chemotherapy and, to a lesser extent, radiotherapy are likely to be offered to most people, whether or not surgery is possible. Specialists advise that the management of pancreatic cancer should be in the hands of a multidisciplinary team including specialists in several aspects of oncology, and is, therefore, best conducted in larger centers.
Surgery

Surgery with the intention of a cure is only possible in around one-fifth (20%) of new cases. Although CT scans help, in practice it can be difficult to determine whether the tumor can be fully removed (its "resectability"), and it may only become apparent during surgery that it is not possible to successfully remove the tumor without damaging other vital tissues. Whether or not surgical resection can be offered depends on various factors, including the precise extent of local anatomical adjacency to, or involvement of, the venous or arterial blood vessels, as well as surgical expertise and a careful consideration of projected post-operative recovery. The age of the person is not in itself a reason not to operate, but their general performance status needs to be adequate for a major operation.
One particular feature that is evaluated is the encouraging presence, or discouraging absence, of a clear layer or plane of fat creating a barrier between the tumor and the vessels. Traditionally, an assessment is made of the tumor's proximity to major venous or arterial vessels, in terms of "abutment" (defined as the tumor touching no more than half a blood vessel's circumference without any fat to separate it), "encasement" (when the tumor encloses most of the vessel's circumference), or full vessel involvement.:22 A resection that includes encased sections of blood vessels may be possible in some cases, particularly if preliminary neoadjuvant therapy is feasible, using chemotherapy:36 and/or radiotherapy.:29–30
Even when the operation appears to have been successful, cancerous cells are often found around the edges ("margins") of the removed tissue, when a pathologist examines them microscopically (this will always be done), indicating the cancer has not been entirely removed. Furthermore, cancer stem




