Robinow Syndrome, Autosomal Dominant 2
A number sign (#) is used with this entry because of evidence that autosomal dominant Robinow syndrome-2 (DRS2) is caused by heterozygous mutation in the DVL1 gene (601365) on chromosome 1p36.
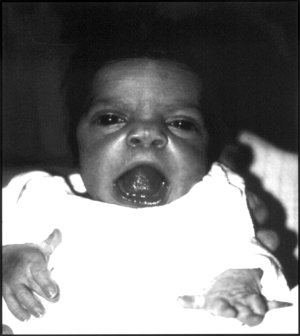
DescriptionRobinow syndrome is a skeletal dysplasia characterized by distinctive facial features, including midface hypoplasia, hypertelorism, a short nose, and a broad mouth, known collectively as 'fetal facies.' Additional features include mesomelic dwarfism, macrocephaly, gingival hypertrophy, dental malocclusion, genital hypoplasia, and brachydactyly (summary by Bunn et al., 2015). Additionally, increased skull bone density and appendicular osteosclerosis are present in patients with DRS2 (White et al., 2015; Bunn et al., 2015).
For a discussion of genetic heterogeneity of Robinow syndrome, see RRS (268310).
Clinical FeaturesSaraiva et al. (1999) reported a pair of monozygotic twin brothers of Portuguese descent with Robinow syndrome. Both had distinctive facial features, including frontal bossing with a larger anterior fontanel, prominent eyes, hypertelorism, small upturned nose, triangular mouth, and gingival hyperplasia. Skeletal anomalies included mesomelic upper limb shortening and short fingers and toes. Each boy also had a small penis and cryptorchidism. At age 14 years, growth and development were normal, and the facial features had become less striking. Saraiva et al. (1999) postulated autosomal dominant inheritance.
Eijkenboom et al. (2012) reported a 17-year-old girl with Robinow syndrome and low-frequency conductive hearing impairment. During childhood she had experienced chronic otitis media with effusion. A perforation of the left tympanic membrane was present. CT scan showed thickening and generalized sclerosis of the calvarium. Exploratory tympanotomy confirmed narrowing of the middle ear cavity, oval and round window niches due to the bony overgrowth, and a thickening and fixation of partially dysmorphic ossicles.
Bunn et al. (2014) reported 2 unrelated patients with Robinow syndrome. A 21-year-old woman had presented at birth with characteristic facial features, brachydactyly, camptodactyly, and clinodactyly. She had chronic otitis media resulting in conductive and sensorineural hearing loss. She also had oligodontia. At age 13 years, she had downslanting palpebral fissures with hypertelorism, midface hypoplasia, wide and depressed nasal bridge, short nose with narrow nares, and gingival hypertrophy. The second patient was a 9-year-old boy born of Latin American parents. At birth he had cleft lip and palate, umbilical hernia, and cryptorchidism. In childhood he was noted to have dysmorphic facial features, including hypertelorism, broad nasal root, flattened nasal tip, narrow nares, gingival hyperplasia, and dental crowding. Radiographs of both patients showed generalized osteosclerosis affecting the skull and axial and appendicular skeleton, undertubulation of the long bones with cortical thickening, and hypoplastic distal phalanges. Serum alkaline phosphatase was increased in 1 patient. Both patients had normal cognitive function.
White et al. (2015) reported 6 unrelated patients with DRS2, as well as the twins originally reported by Saraiva et al. (1999). All had macrocephaly, frontal bossing, high forehead, midface hypoplasia, hypertelorism, short nose, and dental anomalies. Additional common features included prominent eyes, anteverted nares, long philtrum, triangular mouth, thin upper lip, gingival hyperplasia, micrognathia, and abnormal ear shape or position. Skeletal features included mesomelia, brachydactyly, clinodactyly, broad first toes and thumbs, hypoplastic phalanges, and pectus anomalies. Only 2 patients had short stature. Urogenital features such as micropenis and cryptorchidism were present in some patients.
Molecular GeneticsIn 8 patients, including a pair of monozygotic twins reported by Saraiva et al. (1999), with autosomal dominant Robinow syndrome-2, White et al. (2015) identified 6 different de novo heterozygous truncating mutations in the DVL1 gene (see, e.g., 601365.0001-601365.0004). Mutations in the first 3 patients were found by whole-exome sequencing; subsequent patients were identified from a cohort of 62 additional individuals with a similar phenotype who underwent targeted sequencing of the DVL1 gene. Functional studies of the variants were not performed, but studies of 2 patients' cells showed that the truncated proteins were expressed.
In 3 unrelated patients with DRS2 (Eijkenboom et al., 2012 and Bunn et al., 2014), Bunn et al. (2015) identified 3 different heterozygous mutations in the DVL1 gene (601365.0004-610365.0006), all of which resulted in premature termination of the protein and addition of an abnormal, highly basic C-terminal sequence. Analysis of cells from 1 of the patients showed that the mutation (c.1519del; 601365.0004) was translated into a stable protein and not degraded by nonsense-mediated mRNA decay. In vitro cellular expression studies showed that cotransfection of the mutation with wildtype DVL1 resulted in increased canonical WNT (see 164820) activity.
In 17 Robinow syndrome cases from an in-house database, White et al. (2016) performed Sanger sequencing of the penultimate and final exons of the DVL1, DVL2 (602151), and DVL3 (601368) genes and identified heterozygosity for a de novo 1-bp deletion in DVL1 in a Turkish patient. In addition, 4 mutations were detected in the DVL3 gene in 4 probands (see DRS3, 616894). White et al. (2016) stated that the phenotypic features of DVL1- and DVL3-mediated Robinow syndrome were largely concordant, but noted that only 2 of the 4 DVL3-mutated patients for whom clinical information was available had macrocephaly and all 4 had short stature, suggesting that head circumference and stature might be distinguishing features.