Xeroderma Pigmentosum, Complementation Group B
A number sign (#) is used with this entry because the phenotype associated with xeroderma pigmentosum complementation group B (XPB) is caused by mutation in the DNA excision repair gene ERCC3 (133510) on chromosome 2q14.
DescriptionFor a general discussion of xeroderma pigmentosum, see XPA (278700), and of Cockayne syndrome, see CSA (216400).
Cleaver (1990) provided a review of the causes of xeroderma pigmentosum.
Clinical FeaturesLehmann (1982) performed cell fusion studies on cultured cells from 11 patients with Cockayne syndrome. The 11 cell lines were assigned to 3 complementation groups: 2 to group A, 8 to group B, and 1 to group C. The group C patient was thought to have xeroderma pigmentosum also and was the sole known representative of the XP complementation group B. The patient had clinical as well as biologic features of both disorders.
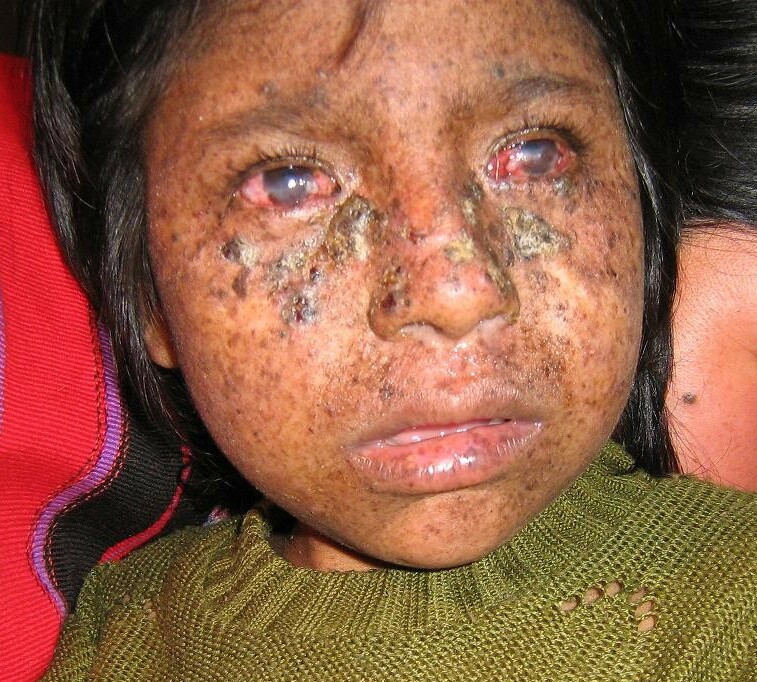
Robbins et al. (1974) reported a young woman with xeroderma pigmentosum complementation group B. She presented with numerous cutaneous malignancies before she was 18 years old. Oh et al. (2006) provided a follow-up of this patient, who was the first identified with XP complementation group B. She had severe features of XP, including extreme sensitivity to sunlight, pigmentation abnormalities, and multiple skin cancers. She also had severe features of Cockayne syndrome, including wizened facial appearance, dwarfism, sensorineural deafness, microcephaly, severe mental retardation, microphthalmia, cataracts, optic atrophy, pigmentary retinal degeneration, hyperreflexia, ataxia, decreased nerve conduction velocities, enlarged cerebral ventricles, basal ganglia calcifications, and immature sexual development. She died at age 33 years of cardiovascular disease.
Scott et al. (1993) described 2 brothers with XP/Cockayne syndrome complex who fell into the complementation group B category. Although UV-induced unscheduled DNA synthesis was only 5% of normal, no skin cancers had appeared in these 38- and 41-year-old brothers. They also had short stature, deafness, pigmentary retinopathy, and neurologic degeneration, but did not have the wizened or cachectic appearance characteristic of the patient reported by Robbins et al. (1974). Vermeulen et al. (1994) presented a table comparing the findings in these brothers with features combining those of XP and CS with patients in the XPD (278730) and XPG (278780) complementation groups.
Oh et al. (2006) reported 2 adult sisters with a relatively mild form of XPB without major manifestations of Cockayne syndrome. They each had XP features of sun sensitivity and freckle-like pigmentation, multiple basal cell carcinomas, and ocular malignant melanomas. The only neurologic manifestations were childhood-onset progressive sensorineural deafness in both and mild cerebellar ataxia in 1. Both sisters were of normal stature and gave birth to healthy children. Studies of patient-derived cells showed decreased DNA repair rates and decreased levels of XPB protein. Genetic analysis identified compound heterozygosity for 2 mutations in the ERCC3 gene (133510.0002; 133510.0004). Each parent was heterozygous for 1 of the mutations.
Molecular GeneticsIn a patient with XPB/Cockayne syndrome reported by Robbins et al. (1974), Weeda et al. (1990) identified a heterozygous mutation in the ERCC3 gene (133510.0001). Oh et al. (2006) identified a second pathogenic ERCC3 mutation (133510.0005) in this patient.
In 2 brothers with XPB/Cockayne syndrome complex reported by Scott et al. (1993), Vermeulen et al. (1994) identified a heterozygous mutation in the ERCC3 gene (133510.0002). Oh et al. (2006) identified a second pathogenic ERCC3 mutation (133510.0008) in these brothers.
Genotype/Phenotype CorrelationsOh et al. (2006) observed a more severe XPB/Cockayne syndrome phenotype in patients with nonsense mutations in the ERCC3 gene on both alleles compared to those with a partially active missense mutation in the ERCC3 gene on at least 1 allele.
PathogenesisHwang et al. (1996) performed detailed in vitro studies of the mutant XPB protein (133510.0001) identified by Weeda et al. (1990). As the XPB protein is a subunit of the generalized transcription factor IIH (GTF2H1; 189972), mutant GTF2H1 isolated from the patient showed decreased 3-prime to 5-prime XPB helicase activity and decreased DNA-dependent ATPase activities, resulting in a severe DNA nucleotide excision repair (NER) defect (5 to 10% of wildtype). There was also evidence for a decrease in basal transcription activity. The patient had combined clinical signs of XP and Cockayne syndrome, which Hwang et al. (1996) concluded were consistent with a combined defect in DNA repair and transcription. The typical XP features such as sun sensitivity, pigmentation abnormalities, and cancer predisposition were consistent with a defect in NER, whereas dwarfism, neuromyelination defects, deafness, and impaired sexual development may have resulted from decreased transcription.