Dermatitis Herpetiformis, Familial
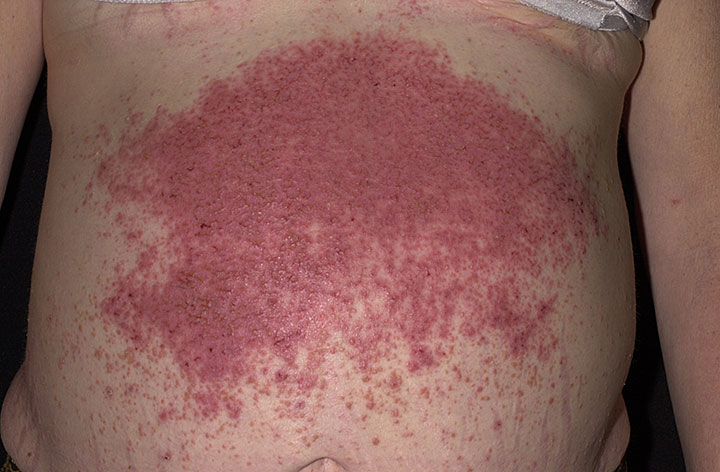
Dermatitis herpetiformis (DH) and celiac disease (CD; 212750) are gluten-sensitive diseases. In classic CD the small intestine is predominantly affected, whereas in DH the skin is also affected, showing typical rash and IgA deposits.
Reunala (1996) reported on the familial incidence of DH in a prospective study started in 1969 in Finland. A total of 1,018 patients with DH were diagnosed and questioned for positive family histories. Of the 999 unrelated DH patients, 105 (10.5%) had 1 or several affected first-degree relatives. Disease in the relatives was either DH (4.4%) or CD (6.1%). Analysis of the 105 families showed that 13.6% of parents, 18.7% of sibs, and 14% of children were affected, a segregation pattern that fitted well to a mendelian dominant mode of inheritance. Gender may also be important because the first-degree relatives affected with DH were more often females and those affected with CD twice as often females as males.
DH and CD have a common immunogenetic background; both disorders are associated with HLA alleles DQA1*0501 (see 146880) and B1*0201 (see 142857). Karell et al. (2002) evaluated the role of the HLA-DQ locus in 25 families in which both classic CD and DH occurred in sibs. By using a family-based approach, they assumed that within each family, variation in environmental factors was substantially lower than in the standard case-control setting, and that the problems related to population stratification could be avoided. Results from Finnish family material comprising 25 discordant and 85 concordant sib pairs, and from case-control material comprising 71 unrelated Hungarian DH and 68 classic CD patients, together indicated that the HLA-DQ locus did not differ between the 2 major outcomes of gluten-sensitive enteropathy. The authors concluded that non-HLA-DR;DQ factors are crucial for the different clinical manifestations of gluten sensitivity.
Using ELISA, Sardy et al. (2002) found that sera from both CD and DH reacted with tissue transglutaminase (TGM2; 190196) and epidermal transglutaminase (TGM3; 600238), but the DH antibodies had a markedly higher avidity for TGM3. Immunofluorescence and confocal microscopy demonstrated that IgA precipitates in the papillary dermis of DH patients contained TGM3, but not keratinocyte transglutaminase (TGM1; 190195) or TGM2. Sardy et al. (2002) concluded that TGM3 is the dominant autoantigen in DH, explaining why skin symptoms rather than intestinal symptoms appear in a proportion of patients with gluten-sensitive disease.
Animal ModelMarietta et al. (2004) developed a mouse model for DH by backcrossing DQ8+ mice lacking endogenous MHC II (Ab0 DQ8+ mice) with NOD mice. Fifteen of 90 NOD DQ8+ mice that were sensitized to gluten developed blistering pathology similar to that seen in DH. Neutrophil infiltration of the dermis, deposition of IgA at the dermal-epidermal junction, and a complete reversal of the blistering phenomenon with the administration of a gluten-free diet with or without dapsone were observed. None of the blistering mice had IgA or IgG endomysial antibodies or IgA antibodies to guinea pig tissue transglutaminase in their sera, and none of 3 blistering mice examined had small bowel pathology.