Nail-Patella Syndrome
Summary
Clinical characteristics.
Nail-patella syndrome (NPS) (previously referred to as Fong's disease), encompasses the classic clinical tetrad of changes in the nails, knees, and elbows, and the presence of iliac horns. Nail changes are the most constant feature of NPS. Nails may be absent, hypoplastic, or dystrophic; ridged longitudinally or horizontally; pitted; discolored; separated into two halves by a longitudinal cleft or ridge of skin; and thin or (less often) thickened. The patellae may be small, irregularly shaped, or absent. Elbow abnormalities may include limitation of extension, pronation, and supination; cubitus valgus; and antecubital pterygia. Iliac horns are bilateral, conical, bony processes that project posteriorly and laterally from the central part of the iliac bones of the pelvis. Renal involvement, first manifest as proteinuria with or without hematuria, occurs in 30%-50% of affected individuals; end-stage renal disease occurs up to 15% of affected individuals. Primary open-angle glaucoma and ocular hypertension occur at increased frequency and at a younger age than in the general population.
Diagnosis/testing.
The diagnosis of nail-patella syndrome is established in a proband with suggestive findings and/or a heterozygous pathogenic variant in LMX1B identified by molecular genetic testing
Management.
Treatment of manifestations: Orthopedic problems may be helped by analgesics, physiotherapy, splinting, bracing, or surgery; MRI of joints to identify abnormal anatomy is important prior to surgery so that appropriate surgical treatment can be planned in advance; ACE inhibitors to control blood pressure and possibly to slow progression of proteinuria; renal transplantation as needed; standard treatment for decreased bone mineral density, hypertension, constipation/inflammatory bowel disease, glaucoma, epilepsy, and dental anomalies.
Surveillance: At least annually: monitoring of blood pressure for hypertension; assessment of urinalysis and first-morning urine albumin:creatinine ratio for renal disease; screening for glaucoma (as soon as a child is compliant). Dental examination at least every six months and DEXA scan as needed.
Agents/circumstances to avoid: Chronic use of NSAIDs because of the detrimental effect on kidney function.
Pregnancy management: The risk of developing preeclampsia may be increased in pregnant women with NPS; hence, frequent urinalysis and blood pressure measurement is recommended during pregnancy. For women taking an ACE inhibitor, transitioning to an alternative treatment ideally prior to pregnancy, or at least as soon as pregnancy is recognized, is recommended to avoid potential adverse effects of ACE inhibitors on the developing fetus.
Genetic counseling.
Nail-patella syndrome is inherited in an autosomal dominant manner. Eighty-eight percent of individuals with NPS have an affected parent; 12% of affected individuals have a de novo pathogenic variant. The offspring of an affected individual are at a 50% risk of inheriting NPS. Prenatal testing and preimplantation genetic testing are possible if the pathogenic variant in the family has been identified.
Diagnosis
Formal clinical diagnostic criteria for nail-patella syndrome (NPS) have not been published, although iliac horns (bilateral, conical, bony processes that project posteriorly and laterally from the central part of the iliac bones of the pelvis) are considered pathognomonic.
Suggestive Findings
Nail-patella syndrome (NPS) should be suspected in individuals with the following clinical and radiologic findings.
Clinical findings
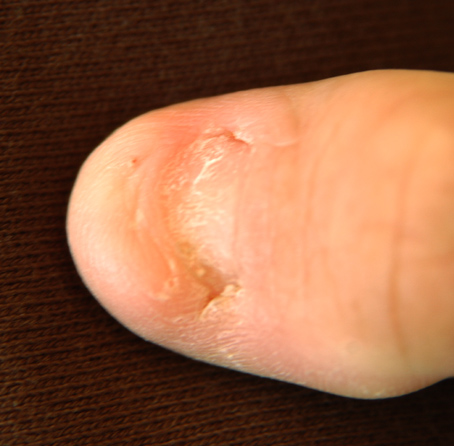
- Nail changes (see Figure 1), including nails that are:
- Absent, hypoplastic, or dystrophic
- Ridged longitudinally or horizontally
- Pitted
- Discolored
- Separated into two halves by a longitudinal cleft or ridge of skin
- Thin or (less often) thickened
- Limited to triangular lunules (lunulae), a characteristic feature of NPS
- Abnormal and unstable patella
- Small, irregularly shaped or absent patella as assessed by palpation or radiographs
- Recurrent subluxation or dislocation of the patella by history and/or physical exam
- Limitation of extension, pronation, and supination at the elbow; cubitus valgus; and antecubital pterygia
 syndrome.">
syndrome.">Figure 1.
Typical presentation of thumb nails (a) and fingernails (b) in nail-patella syndrome. The arrow points to the index finger. Note decrease in severity of nail involvement from second to fifth finger and lack of creases over the distal interphalangeal joints. (more...)
Radiologic findings
- Absent or hypoplastic patella that may be malpositionedNote: Patella ossification centers appear on radiographs between ages three and six years.
- Dysplasia of the radial head, hypoplasia of the lateral epicondyle and capitellum, and prominence of the medial epicondyle
- Iliac horns (bilateral, conical, bony processes that project posteriorly and laterally from the central part of the iliac bones of the pelvis), which are considered pathognomonic of NPS (See Figure 2.)

Figure 2.
Iliac horns (arrows) in an individual with nail-patella syndrome
Family history consistent with an autosomal dominant inheritance pattern. Note: Absence of a known family history of NPS does not preclude the diagnosis.
Establishing the Diagnosis
The diagnosis of nail-patella syndrome is established in a proband with suggestive findings and/or a heterozygous pathogenic variant in LMX1B identified by molecular genetic testing (see Table 1).
Molecular genetic testing approaches can include a combination of gene-targeted testing (single-gene testing and multigene panel) and comprehensive genomic testing (exome sequencing, exome array, genome sequencing) depending on the phenotype.
Gene-targeted testing requires that the clinician determine which gene(s) are likely involved, whereas genomic testing does not. Because the phenotype of nail-patella syndrome is broad, individuals with the distinctive findings described in Suggestive Findings are likely to be diagnosed using gene-targeted testing (see Option 1), whereas those in whom the diagnosis of nail-patella syndrome has not been considered are more likely to be diagnosed using genomic testing (see Option 2).
Option 1
Single-gene testing. Sequence analysis of LMX1B is performed first to detect small intragenic deletions/insertions and missense, nonsense, and splice site variants. Note: Depending on the sequencing method used, single-exon, multiexon, or whole-gene deletions/duplications may not be detected. If no variant is detected by the sequencing method used, the next step is to perform gene-targeted deletion/duplication analysis to detect exon and whole-gene deletions or duplications.
A multigene panel that includes LMX1B and other genes of interest (see Differential Diagnosis) is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. This may be especially useful if renal disease or glaucoma is the predominant presenting feature, as large multigene genetic testing panels for these disease groups are available. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests. For this condition, a multigene panel that includes deletion/duplication analysis is recommended.
For an introduction to multigene panels click here. More detailed information for clinicians ordering genetic tests can be found here.
Option 2
When the diagnosis of nail-patella syndrome has not been considered because an individual has atypical phenotypic features, comprehensive genomic testing (which does not require the clinician to determine which gene is likely involved) may be considered. Exome sequencing is most commonly used; genome sequencing is also possible.
For an introduction to comprehensive genomic testing click here. More detailed information for clinicians ordering genomic testing can be found here.
Additional Testing Considerations for NPS
If targeted genetic testing or exome sequencing are not diagnostic, but NPS is clinically suspected and a dominant inheritance pattern is observed, karyotype may be considered. Chromosomal translocations disrupting LMX1B have also been reported but represent a rare pathogenic mechanism [Duba et al 1998, Silahtaroglu et al 1999, Midro et al 2004].
Table 1.
Molecular Genetic Testing Used in Nail-Patella Syndrome
| Gene 1 | Method | Proportion of Probands with a Pathogenic Variant 2 Detectable by Method |
|---|---|---|
| LMX1B | Sequence analysis 3 | 85% 4 |
| Gene-targeted deletion/duplication analysis 5 | 10% 6 | |
| Karyotype | Rare 7 |
- 1.
See Table A. Genes and Databases for chromosome locus and protein.
- 2.
See Molecular Genetics for information on allelic variants detected in this gene.
- 3.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 4.
Proportion of affected individuals with pathogenic variants identified by sequence analysis of exons 2-6 [Clough et al 1999, Sweeney et al 2003, Harita et al 2020]
- 5.
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications.
- 6.
Dunston et al [2004], Bongers et al [2008]
- 7.
Duba et al [1998], Silahtaroglu et al [1999], Midro et al [2004]
Clinical Characteristics
Clinical Description
The classic clinical tetrad of nail-patella syndrome (NPS) involves changes in the nails, knees, and elbows and the presence of iliac horns (see Diagnosis). Many other features may be seen in NPS, including renal disease and glaucoma [Sweeney et al 2003]. The clinical manifestations are extremely variable in both frequency and severity, with inter- and intrafamilial variability. Individuals may be severely affected by one aspect of NPS but have much milder or no manifestations elsewhere. Though the skeleton is affected in NPS, affected individuals are of average stature.
To date, more than 170 pathogenic variants in LMX1B have been reported in individuals identified to have nail-patella syndrome [Lichter et al 1997, Bongers et al 2002, Sweeney et al 2003, Dunston et al 2005, Lemley 2009, López-Arvizu et al 2011, Boyer et al 2013, Ghoumid et al 2016, Harita et al 2020]. The following description of the phenotypic features associated with this condition is based on these reports.
Table 2.
Select Features of Nail-Patella Syndrome
| Feature | % of Persons with Feature | Comment |
|---|---|---|
| Nail changes | 96%-98% | The thumb nail is most severely affected; the ulnar side of each nail is more severely affected [Sweeney et al 2003, Ghoumid et al 2016]. |
| Digital changes | ~90% | |
| Knee involvement | 74% | Sweeney et al [2003] |
| Elbow involvement | 70% | Sweeney et al [2003] |
| Illiac horns on radiographs | 70%-76% | Pathognomonic for NPS [Tigchelaar et al 2015, Ghoumid et al 2016] |
| Tight Achilles tendons | Unknown prevalence, likely secondary to knee pterygium | May contribute to talipes equinovarus and to-walking |
| Arthrogryposis | Depending on classification of arthrogryposis, ≤75% | Sabir et al [2020] |
| ↓ bone mineral density | Unknown prevalence from limited data, but documented | Towers et al [2005] |
| Renal involvement | 30%-50% | Tigchelaar et al [2015] |
| Ophthalmologic involvement | 10%-25% | Most often presenting as proteinuria, w/or w/o hematuria |
| Gastrointestinal involvement | ~30% | Incl constipation or irritable bowel syndrome |
| ↓ sensation to pain & temperature in hands & feet | ~25% | Sweeney et al [2003], Dunston et al [2005], López-Arvizu et al [2011] |
| Seizures | ~6% | |
| End-stage renal failure | Rare to 15% | Price et al [2018], Harita et al [2020] |
| Vascular anomalies | Rare | Unclear if this is a rare co-occurrence or part of NPS |
NPS = nail-patella syndrome
Nail changes are the most constant feature of NPS.
- Nail changes may be observed at birth and are most often bilateral and symmetric.
- The thumbnails are the most severely affected; the severity of the nail changes tends to decrease from the index finger toward the little finger.
- Each individual nail is usually more severely affected on its ulnar side.
- Dysplasia of the toenails is usually less marked and less frequent than that of the fingernails; if the toenails are involved, it is often the fifth toenail that is affected.
Digital changes. A reduction in flexion of the distal interphalangeal (DIP) joints is associated with loss of the creases in the skin overlying the dorsal surface of the DIP joints of the fingers.
- The gradient of severity is the same as seen in the nails; therefore, the index fingers are the most affected.
- Hyperextension of the proximal interphalangeal (PIP) joints with flexion of the DIP joints (resulting in "swan-necking") and fifth-finger clinodactyly may also be seen.
Knee abnormalities. Symptoms include pain, instability, locking, clicking, patella dislocation, and inability to straighten the knee joint. Knee involvement may also be associated with poor development of the vastus medialis muscle.
- Patellae:
- Findings may be asymmetric.
- The patellae may be small, irregularly shaped, or absent.
- The displacement of the patella is lateral and superior; the hypoplastic patella is often located laterally and superiorly even when not actually dislocated.
- There may be prominent medial femoral condyles, hypoplastic lateral femoral condyles, and prominent tibial tuberosities.
- These changes together with a hypoplastic or absent patella give the knee joint a flattened profile.
- Flexion contractures of the knees may occur due to tight hamstring muscles
- Osteochondritis dissecans, synovial plicae, and absence of the anterior cruciate ligament may also occur.
- Early degenerative arthritis is common.
Elbow involvement can include:
- Limitation of extension, pronation, and supination at the elbow
- Cubitus valgus
- Antecubital pterygia
Affected individuals may experience dislocation of the radial head, usually posteriorly. Elbow involvement may be asymmetric.
Illiac horns are considered pathognomonic of NPS [Sweeney et al 2003].
- Pelvic x-ray is usually necessary for their detection (Figure 2).
- Although large horns may be palpable, they are typically asymptomatic.
- Iliac horns may be seen on third-trimester ultrasound scanning [Feingold et al 1998], on x-ray at birth, and by bone scan [Goshen et al 2000].
- In children, iliac horns may have an epiphysis at the apex.
Involvement of the ankles and feet
- Talipes equinovarus, calcaneovarus, calcaneovalgus, equinovalgus, and hyperdorsiflexion of the foot may occur.
- Tight Achilles tendons are common, contributing to talipes equinovarus and to toe-walking.
- Pes planus is common.
Arthrogryposis. Though contractures of the elbows, knees, and calcaneovarus/calcaneovalgus are recognized in individuals with NPS, the term "arthrogryposis" is not often used in the clinical description of this condition.
- Sabir et al [2020] noted "arthrogryposis" as the presenting feature of an affected individual who underwent whole-exome sequencing.
- Due to limitations of phenotype search terms associated with established gene variants, LMX1B and NPS were not considered.
- By definition, arthrogryposis refers to multiple congenital, usually non-progressive joint contractures involving more than one joint; therefore, many people with NPS may be considered to have arthrogryposis (suggested by Sabir et al [2020] to be present in as many as 75% of individuals with NPS).
Spinal and chest wall problems. Back pain occurs in half of individuals with NPS. There may be an increased lumbar lordosis, scoliosis (usually mild), spondylolisthesis, spondylolysis, or pectus excavatum.
Osteoporosis. Bone mineral density is reduced by 8%-20% in the hips of individuals with NPS. An increased rate of fractures has also been reported.
General appearance. A lean body habitus may be associated with NPS and affected individuals often have difficulty putting on weight (particularly muscle) despite adequate dietary intake and exercise.
- In particular, muscle mass in the upper arms and upper legs tends to be decreased.
- The tendency to be very lean is most evident in adolescents and young adults and becomes less apparent after middle age.
- Increased lumbar lordosis may make the buttocks appear prominent.
- The high forehead and hairline, particularly at the temples, resembles a receding male pattern hairline when seen in women.
Renal involvement
- Renal involvement is present in 30%-50% of individuals with NPS. Variable rates of end-stage renal disease (ESRD) have been described as high as 15% by Lemley [2009], 5% by Sweeney et al [2003], and more recently, less than 5% by Harita et al [2020].
- The first sign of renal involvement is usually proteinuria, with or without hematuria.
- Proteinuria may present at any age from birth onwards and may be intermittent.
- Renal problems may present during (or be exacerbated by) pregnancy.
- Once proteinuria is present, it may remit spontaneously, remain asymptomatic, or progress to nephrotic syndrome and occasionally to ESRD.
- Steroids may not be effective in the treatment of proteinuria in individuals with NPS [Nakata et al 2017, Harita et al 2020].
- Progression to renal failure may appear to occur rapidly or after many years of asymptomatic proteinuria. The factors responsible for this progression are yet to be identified but the presence and severity of proteinuria appears to be predictive of progression [Harita et al 2020]. In individuals with ESRD, renal transplantation may be considered and typically has a favorable outcome (see Management, Treatment of Manifestations).
- Nephritis may also occur in NPS.
- Ultrastructural (electron microscopic) renal abnormalities are the most specific histologic changes seen in individuals with NPS and include irregular thickening of the glomerular basement membrane with electron-lucent areas giving a mottled "moth-eaten" appearance, and the presence of collagen-like fibers within the basement membrane and the mesangial matrix.
Ophthalmologic findings
- Primary open-angle glaucoma and ocular hypertension occur at increased frequency in NPS and at a younger age than in the general population [Lichter et al 1997, Sweeney et al 2003, Ghoumid et al 2016].
- Congenital and normal-tension glaucoma have also been reported in individuals with NPS [Lichter et al 1997].
- Iris pigmentary changes (termed Lester's sign) consisting of a zone of darker pigmentation shaped like a cloverleaf or flower around the central part of the iris are seen frequently.
Gastrointestinal involvement. One third of individuals with NPS have problems with constipation (often from birth) or irritable bowel syndrome [Sweeney et al 2003].
Neurologic problems. Many individuals with NPS exhibit reduced sensation to pain and temperature in the hands and feet, most likely because of the inability of Aδ and C fibers to connect with interneurons in the dorsal spinal cord [Dunston et al 2005]. Some affected individuals report intermittent numbness, tingling, and burning sensations in the hands and feet, with no obvious precipitant.
- Rarely, these symptoms may be secondary to local orthopedic problems or neurologic compromise from the spine or cervical ribs.
- In most cases, the paresthesia follows a glove and stocking pattern rather than the distribution of a particular dermatome or peripheral nerve.
Epilepsy was reported in 6% of affected individuals in one large study [Sweeney et al 2003].
Dental problems. Dental problems may include weak, crumbling teeth and thin dental enamel [Sweeney et al 2003].
Vasomotor problems. Some individuals have symptoms of a poor peripheral circulation, such as very cold hands and feet, even in warm weather. Some may be diagnosed with Raynaud's phenomenon [Sweeney et al 2003].
Vascular anomalies. There are limited reports of vascular anomalies in individuals with NPS, including internal carotid artery aplasia [Kraus et al 2020] and spontaneous coronary artery dissection [Nizamuddin et al 2015, Kaadan et al 2018]. The prevalence of vascular issues in a large population of individuals with NPS and the function of LMX1B in vessel formation must be studied further to determine if these findings are rare coincidental occurrences or part of the NPS phenotype. At this point, it is too early to know if this is part of the NPS phenotype.
Genotype-Phenotype Correlations
The majority (~80%) of pathogenic variants in LMX1B are found in the LIM domains.
Renal manifestations. Bongers el al [2005] suggested that individuals with a pathogenic variant in LMX1B in the LMX1 homeodomain showed significantly more frequent and higher values of proteinuria compared to those with pathogenic variants in the LIM domains. This observation is supported by Harita et al [2020] in a cohort of Japanese individuals with NPS. Thus far, it is not possible to predict progression of renal manifestations to end-stage renal disease based on genotype alone due to inter-individual variability. However the presence and severity of proteinuria appear to correlate with progression of renal disease.
Non-renal manifestations. No clear genotype-phenotype association is apparent for extrarenal manifestations of NPS.
Nomenclature
Nail-patella syndrome is the most accepted term but has the disadvantage of implying that nail and patellar dysplasia are the most important features. Hereditary onycho-osteodysplasia (HOOD) may be more accurate, but is rarely used. Perhaps hereditary onycho-osteodysplasia with nephropathy and glaucoma would be the best term.
The terms Fong's disease and Turner syndrome have also been used.
- Captain EE Fong described the presence of unusual horn-like anomalies on the posterior aspect of the iliac bones in a woman undergoing an intravenous pyelogram. Fong published the description in 1946 and although he did not associate the anomaly with nail-patella syndrome, his name was connected to this condition [Fong 1946].
- Turner* and Keiser published earlier descriptions of the iliac horns in individuals with nail-patella syndrome in 1933 and 1939, respectively.*Note: Referring to JW Turner and not HH Turner, who described the phenotype associated with a 45,X karyotype
Prevalence
The prevalence of NPS has been roughly estimated at 1:50,000 but may be higher because of undiagnosed individuals with a mild phenotype.
Differential Diagnosis
Table 3a.
Genes of Interest in the Differential Diagnoses of Nail-Patella Syndrome
| Gene(s) | Disorder | MOI | Features of Differential Diagnosis Disorder | |
|---|---|---|---|---|
| Overlapping w/NPS | Distingusihing from NPS | |||
| ARID1A ARID1B SMARCA4 SMARCB1 SMARCE1 SOX11 | Coffin-Siris syndrome | AD |
|
|
| CDC6 CDC45 CDT1 GMNN MCM5 ORC1 ORC4 ORC6 | Meier-Gorlin syndrome (OMIM PS224690) | AR AD |
|
|
| KAT6B | Genitopatellar syndrome (See KAT6B Disorders.) | AD |
|
|
| RECQL4 | RAPADILINO syndrome 1 (OMIM 266280) | AR |
|
|
| TBC1D24 | DOORS syndrome (See TBC1D24-Related Disorders.) | AR | Absent or poorly formed nails |
|
| TBX4 | Small patella syndrome (ischiopatellar dysplasia, coxopodo-patellar syndrome) (OMIM 147891) | AD |
|
|
AD = autosomal dominant; AR = autosomal recessive; MOI = mode of inheritance; NPS = nail-patella syndrome
- 1.
See Rothmund-Thomson Syndrome, Genetically Related Disorders.
Table 3b.
Chromosome Disorder and Hereditary Disorders of Unknown Genetic Cause in the Differential Diagnoses of Nail-Patella Syndrome
| Disorder | Features of Differential Diagnosis Disorder | |
|---|---|---|
| Overlapping w/NPS | Distinguishing from NPS | |
| Brachymorphism- onychodysplasia- dysphalangism syndrome (OMIM 113477) | Small nails | |