Axenfeld-Rieger Syndrome, Type 3
A number sign (#) is used with this entry because Axenfeld-Rieger syndrome type 3 (RIEG3) is caused by heterozygous mutation in the FOXC1 gene (601090) on chromosome 6p25.
For a general phenotypic description and a discussion of genetic heterogeneity of Axenfeld-Rieger syndrome, see RIEG1 (180500).
See also chromosome 6pter-p24 deletion syndrome (612582), which shows phenotypic overlap with Axenfeld-Rieger syndrome type 3.
Clinical FeaturesGould et al. (1997) examined 13 members of a 4-generation family segregating autosomal dominant Axenfeld-Rieger anomaly and identified 7 affected individuals who had a prominent and anteriorly displaced Schwalbe line, iris stromal hypoplasia, and corectopia (anterior segment dysgenesis); nonocular features of Axenfeld-Rieger syndrome, including jaw, dental, and umbilical anomalies, were not present. However, Mears et al. (1998) later identified affected members of this family who had heart anomalies and hearing loss.
Cunningham et al. (1998) described an autosomal dominant syndrome that combined familial Axenfeld-Rieger anomaly with atrial septal defect and sensorineural hearing loss. The 30-year-old proposita was first seen at the age of 6 months with increased intraocular pressures. Her 22-year-old half brother presented with glaucoma at 10 months of age. He was referred for evaluation of murmur and a failure to thrive and was found to have atrial septal defect requiring surgical repair. The proposita's 50-year-old father was also seen in the Wilmer Ophthalmological Institute of the Johns Hopkins Hospital in early infancy with increased intraocular pressures. Failure of both medical and surgical treatment resulted in enucleation of a blind, painful left eye at 10 years of age. Independently, the father was found to have an atrial septal defect at 11 months of age, which eventually required surgical repair. The deceased paternal grandmother of the proposita had been seen in the Wilmer Institute at 38 years of age with end-stage glaucoma and central retinal vein occlusion of the left eye that required enucleation. Thus, 3 generations appeared to have been affected, with 1 instance of male-to-male transmission and an affected child from each of 2 unaffected mothers. The proposita had normal hearing and no evidence of cardiac abnormality. The father was described as having marked bilateral sensorineural hearing loss; his son had moderate sensorineural hearing loss bilaterally. Craniofacial and dental development was normal in all.
Baruch and Erickson (2001) described a brother and sister with Axenfeld-Rieger anomaly, hypertelorism, clinodactyly, and cardiac anomalies. The older sib had a sensorineural hearing loss and wore hearing aids; he also underwent surgical correction of a 'very large' patent ductus arteriosus and a diaphragmatic hernia. His sister had a large patent ductus arteriosus that closed spontaneously and a moderate atrial septal defect. The authors noted similarities between the phenotype in these sibs and the disorder described by Cunningham et al. (1998).
Grosso et al. (2002) described a family with Axenfeld-Rieger anomaly, cardiac malformations (tricuspid and mitral valve defects), and sensorineural hearing loss. The proband was more severely affected than her father, who was more severely affected than his father. Karyotyping of the proband was normal. A number of other relatives were not available for examination but by report had variable features. No family members had craniofacial anomalies, dental hypoplasia, or involuted periumbilical skin.
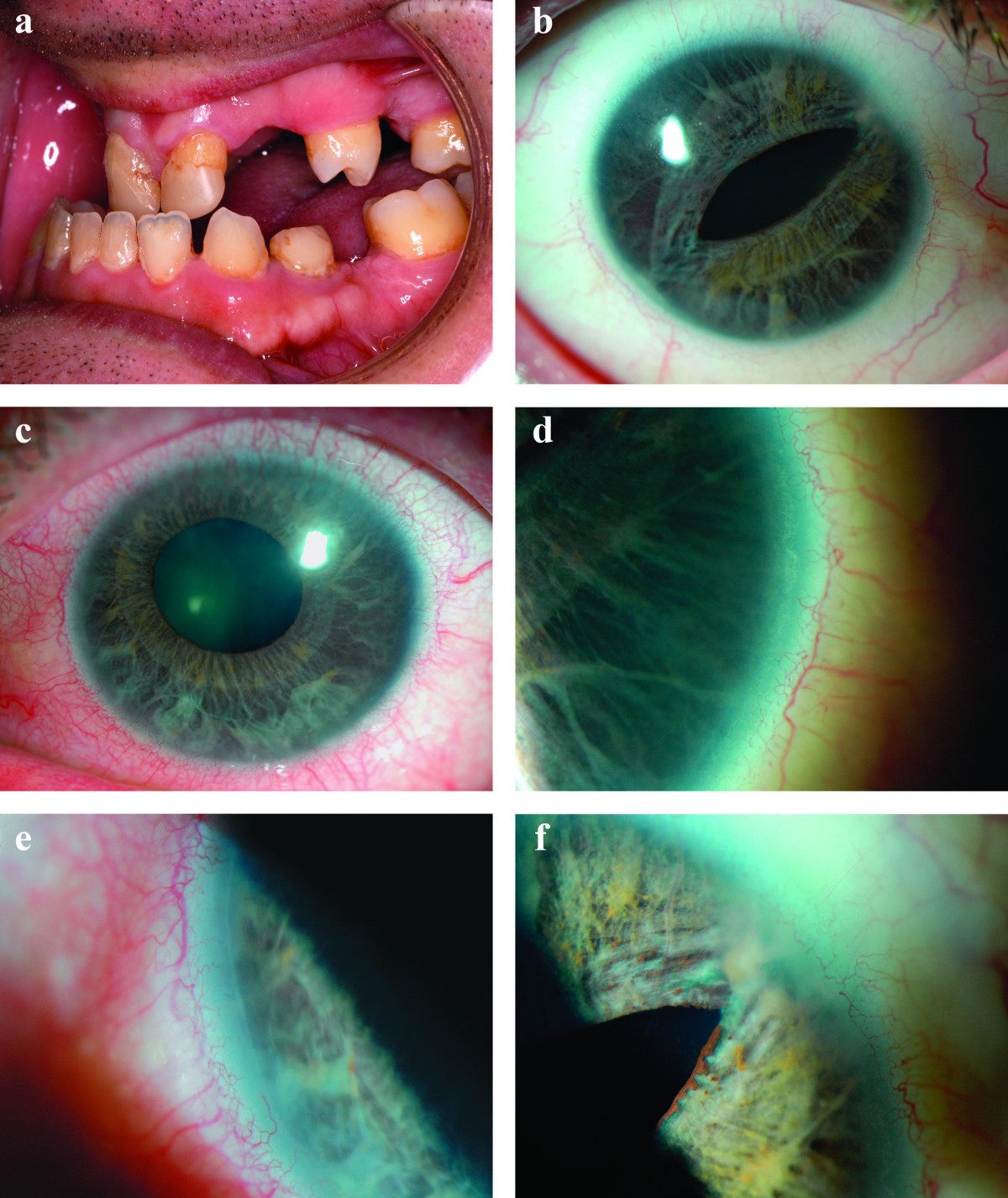
Weisschuh et al. (2008) studied 5 affected members of a 3-generation family with Axenfeld-Rieger syndrome, all of whom had a displaced Schwalbe line, iridocorneal adhesions, iris hypoplasia, and glaucoma. One individual also had corectopia. The 15-year-old female proband had been diagnosed at birth with Peters anomaly, which was confirmed by ophthalmoscopic examination under anesthesia; gonioscopy, which could only be performed on her right eye, showed aniridia with a 1-mm iris rudiment. Extraocular anomalies were present in all 5 patients: 3 had maxillary hypoplasia, 2 had protuberant umbilical skin, 2 had ureteral stenosis, 1 had hypertelorism, and 1 had atrial septal defect.
CytogeneticsIn 2 sibs with Axenfeld-Rieger anomaly, hypertelorism, and cardiac anomalies, Baruch and Erickson (2001) found an unbalanced translocation der(6)t(6;8)(p25.1;q24.23), making them monosomic for 6pter-p25.1 and trisomic for 8q24.23-qter.
MappingGould et al. (1997) performed linkage analysis in a 4-generation family segregating Axenfeld-Rieger anomaly, later found to segregate Axenfeld-Rieger syndrome (Mears et al., 1998), and obtained a maximum lod score of 3.1 (theta = 0) at marker D6S344 on chromosome 6p25. A recombination event defined a 6.4-cM critical interval between D6S1600 and D6S1617.
Molecular GeneticsIn the family originally described by Gould et al. (1997) with Axenfeld-Rieger anomaly, Mears et al. (1998) also reported deafness and heart anomalies and identified a missense mutation in the FOXC1 gene (S82T; 601090.0008).
In 9 affected individuals over 3 generations of a family with Axenfeld-Rieger syndrome, Mirzayans et al. (2000) identified heterozygosity for a nonsense mutation (E23X; 601090.0005) in the FOXC1 gene. Affected individuals presented with a variable degree of iris hypoplasia, displaced pupils (corectopia), and a prominent, anteriorly displaced Schwalbe line (posterior embryotoxon) to which peripheral iris strands were attached bridging the iridocorneal angle. Glaucoma was observed in 1 individual. Extraocular features included hypertelorism in 5 patients, microdontia in 4, flat midface in 4, umbilical abnormalities in 2, cardiac defect in 1, and hearing loss in 1.
In a mother and son with Axenfeld-Rieger syndrome, Ito et al. (2007) analyzed the FOXC1 gene and identified a missense mutation (601090.0010) that was de novo in the mother. The 2-month-old boy had corectopia and hypertelorism and slight excess of skin at the umbilicus; ophthalmologic examination revealed a posterior embryotoxon and an intraocular pressure of 14 mm Hg, with abnormally high cup-disc ratios (0.85 and 0.80 for left and right eyes, respectively). The 27-year-old mother was subsequently examined and found to have iris hypoplasia, a prominent Schwalbe line, and peripheral anterior synechiae, but no glaucoma; she had no dental or facial abnormalities. The eye findings were bilateral in both patients.
In 5 affected members of a 3-generation family with Axenfeld-Rieger syndrome, who displayed a substantial degree of intrafamilial phenotypic variability including Peters anomaly in 1 patient, Weisschuh et al. (2008) identified heterozygosity for a nonsense mutation in the FOXC1 gene (601090.0011). The authors also screened the PITX2 (601542) and CYP1B1 (601771) genes in this family and identified no disease-causing mutations, although they did find that 2 known functional polymorphisms in CYP1B1, V432L and N453S, were carried in heterozygosity by all affected individuals except for the proband, who was homozygous for the common N453 allele, and her brother, who was homozygous for the minor 432L allele.
Aldinger et al. (2009) analyzed brain imaging studies in 3 patients from 2 families with missense mutations in FOXC1 resulting in Axenfeld anomaly (601090.0003) and Axenfeld-Rieger syndrome type 3 (601090.0008), respectively, previously reported by Nishimura et al. (1998), Gould et al. (1997), and Mears et al. (1998), and observed mild cerebellar vermis hypoplasia and an abnormal white matter signal corresponding to prominent perivascular spaces; 1 of the patients also showed meningeal defects. Aldinger et al. (2009) concluded that partial loss of FOXC1 function results in cerebellar malformation.
Chanda et al. (2008) analyzed the breakpoint architecture in 10 pedigrees with duplications or deletions at chromosome 6p25 and a diagnosis of glaucoma associated with iris hypoplasia or Axenfeld-Rieger syndrome, and found that in contrast to most previous examples, the majority of the segmental duplications and deletions utilized coupled homologous and nonhomologous recombination mechanisms. A junction fragment from a pedigree with Axenfeld-Rieger syndrome and glaucoma, previously studied by Lehmann et al. (2002), exhibited an unprecedented 367-bp insert derived from tandemly arranged breakpoint elements that was unassociated with template switching and occurred in a segmental deletion. Chanda et al. (2008) stated that their results extended the mechanisms involved in structural variant formation and provided strong evidence that a spectrum of recombination, DNA repair, and replication underlie chromosome 6p25 rearrangements.
Genotype/Phenotype CorrelationsUsing patient records and clinical questionnaires, Strungaru et al. (2007) reviewed 126 patients diagnosed with Axenfeld-Rieger malformation or syndrome, representing 20 probands, in whom mutations in the PITX2 or FOXC1 genes had been identified. The authors found that 75% of these patients had glaucoma that developed in adolescence or early adulthood, and that patients with PITX2 defects or FOXC1 duplications had a more severe prognosis for glaucoma development than patients with FOXC1 mutations; only 18% of PITX2- or FOXC1-related cases of glaucoma responded to surgical and/or medical therapy. In addition, patients with nonocular findings were more likely to have defects in PITX2 than FOXC1.