Xeroderma Pigmentosum, Complementation Group F
A number sign (#) is used with this entry because of evidence that the disorder is caused by homozygous or compound heterozygous mutation in the ERCC4 gene (133520) on chromosome 16p13.
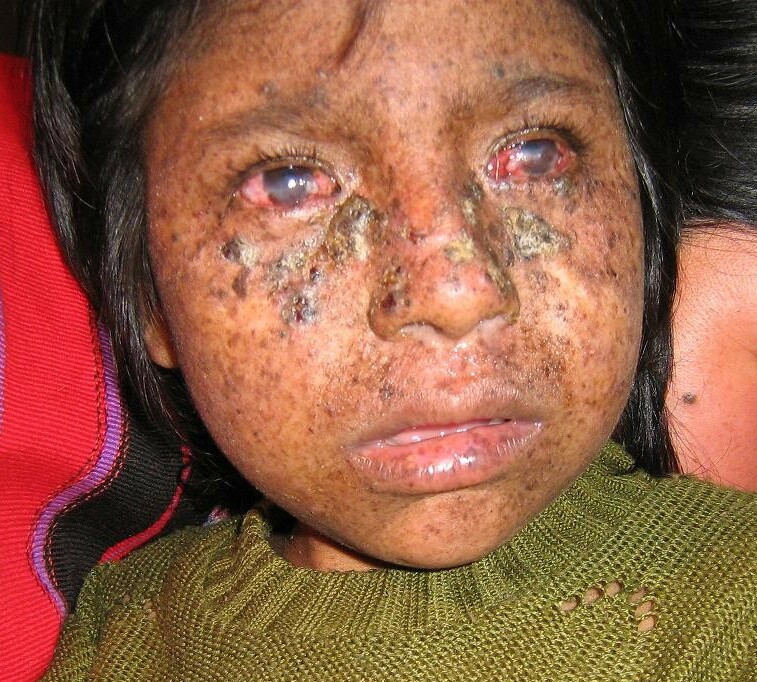
DescriptionXeroderma pigmentosum is an autosomal recessive disorder characterized by sun sensitivity and increased skin sensitivity to UV light, as well as an increased risk of skin cancer associated with a defect in nucleotide excision repair (NER). The XPF form of XP is usually relatively mild compared to other forms. Patients with XPF tend to have later onset of skin cancer. Some patients with XPF may develop neurologic impairment or growth defects, and are then classified as having Cockayne syndrome (summary by Kashiyama et al., 2013).
For a general phenotypic description and a discussion of genetic heterogeneity of xeroderma pigmentosa, see XPA (278700), and of Cockayne syndrome, see CSA (216400).
Clinical FeaturesGroup F xeroderma pigmentosum had probably been observed only in Japan (Fujiwara et al., 1985) until the report by Norris et al. (1988) of a case in an English woman. The patient reported by Norris et al. (1988) was a 22-year-old-white woman with mild cutaneous changes, no tumors, and normal sensitivity to monochromatic ultraviolet irradiation. Unscheduled DNA synthesis in cultured fibroblasts after UV irradiation was reduced to 13% of controls during the first 2 hours and rose to 45% of normal by 7 to 8 hours. The findings were consistent with a defect in nucleotide excision repair (NER).
Yamamura et al. (1989) described a 61-year-old female patient with XP assigned to complementation group F by the cell fusion-complementation method. Her fibroblasts in culture exhibited a defective DNA repair capacity of 10 to 15% unscheduled DNA synthesis and a 3-fold increased sensitivity to the lethal effects of ultraviolet light. The patient had mild clinical symptoms consisting of numerous pigmented freckles and a small number of seborrheic keratosis-like papules. She had no skin cancers in the sun-exposed areas and no neurologic abnormalities. Yamamura et al. (1989) reviewed 11 Japanese group F patients demonstrating very mild skin symptoms and no ocular or neuropsychiatric abnormalities. Single skin cancers occurred in only 3 of the 11 patients, with an average age of 52 years for their first skin malignancy.
Cockayne Syndrome
Moriwaki et al. (1993) reported a 48-year-old Japanese man with xeroderma pigmentosum associated with mental retardation, cerebral atrophy, and cerebellar ataxia. Genetic complementation tests by cell fusion with polyethylene glycol revealed that the patient belonged to group F. He died of bile duct cancer at the age of 50. Moriwaki et al. (1993) stated that this was the first report of an XPF patient with neurologic abnormalities.
Sijbers et al. (1998) reported a Caucasian patient with XPF. He had mild ocular photophobia from childhood and acute skin reactions occurred upon exposure to sunlight. After age 27, he developed several basal and squamous cell carcinomas. In his late forties, he developed progressive neurologic symptoms, including intellectual decline, mild chorea and ataxia, and marked cerebral and cerebellar atrophy. Sijbers et al. (1998) stated that such neurologic abnormalities were unusual in XPF, having been described in only 1 of 17 other XPF individuals. The patient's 5-fold reduced activity of nucleotide excision repair in cultured cells, combined with moderately affected cell survival and DNA replication after UV exposure, was typical of XPF.
Kashiyama et al. (2013) reported a 16-year-old boy (CS1USAU) with Cockayne syndrome. He developed normally for the first year without obvious abnormalities, except for the microcephaly. At age 5 years, he developed multiple unusual plantar warts on his hands and forearms, unusual freckling, and sun sensitivity. Examination at age 7 years showed short stature, poor growth, and microcephaly. He had deep-set eyes, progressive scoliosis, and multiple contractures in his feet; he required lengthening of the Achilles tendon because of muscle cramps in his hamstrings and calves. His skin was deeply pigmented with rashes and flat freckles. Other features included hearing impairment, learning disabilities, attention deficit-hyperactivity disorder, migraines, and feeding difficulties. Brain MRI showed some delayed myelination and basal ganglia shortening. A second unrelated patient (XPCS1CD) had a more complex phenotype comprising XPF, Cockayne syndrome, and Fanconi anemia (see FANCQ; 615272). She showed intrauterine growth failure and later had short stature and microcephaly. Development was globally delayed. She was noted to have sun sensitivity with severe sunburns at age 18 months, and was carefully photoprotected. She developed sensorineural hearing loss at age 5 years, followed by progressive ataxia, tremor, weakness, and nystagmus. Between ages 6 and 9 years, she became pancytopenic, consistent with bone marrow failure, and at age 10, she developed hypertension and severe proteinuria, consistent with renal failure. Examination at age 11 showed microcephaly, short stature, deep-set eyes, prominent nose, small teeth, and freckling over the nose and cheeks. The skin had an aged appearance, and palmar erythema, plane warts, and cafe-au-lait macules were present. Liver function was normal; renal biopsy showed focal segmental glomerular sclerosis, thickening of the capillary basement membrane, interstitial fibrosis, and tubular atrophy. She died of renal failure at age 12 years. Dermal fibroblasts from both patients showed significantly decreased RNA synthesis activity compared to controls, indicating a deficiency in transcription-coupled NER (TC-NER), as expected for Cockayne syndrome cells, as well as a decrease in unscheduled DNA synthesis, indicating a defect in global genome NER (GG-NER). Complementation studies showed that the 2 patients could be assigned to group XPF. Both cell lines were also sensitive to the crosslinking agent mitomycin-C, indicating a defect in the repair of DNA interstrand crosslinks (ICL).
MappingSaxon et al. (1989) found that microcell-mediated transfer of a single human chromosome from repair-proficient human cells to XP cells of complementation group F resulted in partial complementation of repair-defective phenotypes. They identified the complementing chromosome as human chromosome 15 by cytogenetic and molecular analysis. About 20% of the resistance of wildtype cells to killing by UV radiation or by the UV-mimetic chemical 4-nitroquinoline-1-oxide, as well as partial repair synthesis of DNA measured as unscheduled DNA synthesis, resulted from the transfer of the chromosome. Although the work seemed to indicate that the gene on chromosome 15 carries a mutation determining XPF, the reason for the incomplete correction was unclear. Because of incomplete correction, the assignment of XPF to chromosome 15 was considered in limbo (Cleaver, 1993).
By means of fluorescence in situ hybridization, Sijbers et al. (1996) mapped the XPF gene to 16p13.2-p13.1. This corresponds to the location of ERCC4, a human repair gene complementing rodent nucleotide excision repair mutants of group 4, that was originally identified using cell hybrids (Liu et al., 1993) and a genomic clone (Thompson et al., 1994).
Molecular GeneticsIn the patient with XPF reported by Norris et al. (1988), Sijbers et al. (1996) identified compound heterozygous mutations in the ERCC4 gene (133520.0001 and 133520.0002).
In a patient with XPF and late-onset neurologic features, Sijbers et al. (1998) identified a homozygous mutation in the ERCC4 gene (R788W; 133520.0002).
Cleaver et al. (1999) tabulated the mutations that had been reported in the XPF gene. These included 8 mutations reported by Matsumura et al. (1998). Their patients varied in age from 22 to 73 years. The XPF complementation group is rare and the majority of cases have been found in Japan.
In 2 unrelated patients with XPF and Cockayne syndrome, Kashiyama et al. (2013) identified compound heterozygous mutations in the ERCC4 gene (133520.0008-133520.0010).
Animal ModelMunoz et al. (2005) found that transgenic mice that overexpress the telomere-binding protein TRF2 (602027) had a severe phenotype in the skin in response to light, consisting of premature skin deterioration, hyperpigmentation, and increased skin cancer, resembling xeroderma pigmentosum. Keratinocytes from these mice were hypersensitive to ultraviolet irradiation and DNA crosslinking agents. The skin cells of these mice had marked telomere shortening, loss of the telomeric G-strand overhang, and increased chromosomal instability. Munoz et al. (2005) found that telomere loss in these mice was mediated by XPF. The findings suggested that TRF2 provides a crucial link between telomere function and ultraviolet-induced damage repair, whose alteration underlies genomic instability, cancer, and aging.