Lyme Disease

Lyme disease, also known as Lyme borreliosis, is an infectious disease caused by the Borrelia bacterium which is spread by ticks. The most common sign of infection is an expanding red rash, known as erythema migrans, that appears at the site of the tick bite about a week after it occurred. The rash is typically neither itchy nor painful. Approximately 70–80% of infected people develop a rash. Other early symptoms may include fever, headache and tiredness. If untreated, symptoms may include loss of the ability to move one or both sides of the face, joint pains, severe headaches with neck stiffness, or heart palpitations, among others. Months to years later, repeated episodes of joint pain and swelling may occur. Occasionally, people develop shooting pains or tingling in their arms and legs. Despite appropriate treatment, about 10 to 20% of people develop joint pains, memory problems, and tiredness for at least six months.
Lyme disease is transmitted to humans by the bites of infected ticks of the genus Ixodes. In the United States, ticks of concern are usually of the Ixodes scapularis type, and must be attached for at least 36 hours before the bacteria can spread. In Europe, ticks of the Ixodes ricinus type may spread the bacteria more quickly. In North America, the bacteria Borrelia burgdorferi and Borrelia mayonii cause Lyme disease. In Europe and Asia, Borrelia afzelii and Borrelia garinii are also causes of the disease. The disease does not appear to be transmissible between people, by other animals, or through food. Diagnosis is based upon a combination of symptoms, history of tick exposure, and possibly testing for specific antibodies in the blood. Blood tests are often negative in the early stages of the disease. Testing of individual ticks is not typically useful.
Prevention includes efforts to prevent tick bites such as by wearing clothing to cover the arms and legs, and using DEET-based insect repellents. Using pesticides to reduce tick numbers may also be effective. Ticks can be removed using tweezers. If the removed tick was full of blood, a single dose of doxycycline may be used to prevent development of infection, but is not generally recommended since development of infection is rare. If an infection develops, a number of antibiotics are effective, including doxycycline, amoxicillin, and cefuroxime. Standard treatment usually lasts for two or three weeks. Some people develop a fever and muscle and joint pains from treatment which may last for one or two days. In those who develop persistent symptoms, long-term antibiotic therapy has not been found to be useful.
Lyme disease is the most common disease spread by ticks in the Northern Hemisphere. It is estimated to affect 300,000 people a year in the United States and 65,000 people a year in Europe. Infections are most common in the spring and early summer. Lyme disease was diagnosed as a separate condition for the first time in 1975 in Old Lyme, Connecticut. It was originally mistaken for juvenile rheumatoid arthritis. The bacterium involved was first described in 1981 by Willy Burgdorfer. Chronic symptoms following treatment are well described and are known as "post-treatment Lyme disease syndrome" (PTLDS). PTLDS is different from chronic Lyme disease, a term no longer supported by the scientific community and used in different ways by different groups. Some healthcare providers claim that PTLDS is caused by persistent infection, but this is not believed to be true because no evidence of persistent infection can be found after standard treatment. A vaccine for Lyme disease was marketed in the United States between 1998 and 2002, but was withdrawn from the market due to poor sales. Research is ongoing to develop new vaccines.
Signs and symptoms

Lyme disease can affect multiple body systems and produce a broad range of symptoms. Not everyone with Lyme disease has all of the symptoms, and many of the symptoms are not specific to Lyme disease but can occur with other diseases as well.
The incubation period from infection to the onset of symptoms is usually one to two weeks, but can be much shorter (days), or much longer (months to years). Lyme symptoms most often occur from May to September, because the nymphal stage of the tick is responsible for most cases. Asymptomatic infection exists, but occurs in less than 7% of infected individuals in the United States. Asymptomatic infection may be much more common among those infected in Europe.
Early localized infection
Early localized infection can occur when the infection has not yet spread throughout the body. Only the site where the infection has first come into contact with the skin is affected. The initial sign of about 80% of Lyme infections is an Erythema migrans (EM) rash at the site of a tick bite, often near skin folds, such as the armpit, groin, or back of knee, on the trunk, under clothing straps, or in children's hair, ear, or neck. Most people who get infected do not remember seeing a tick or the bite. The rash appears typically one or two weeks (range 3–32 days) after the bite and expands 2–3 cm per day up to a diameter of 5–70 cm (median 16 cm). The rash is usually circular or oval, red or bluish, and may have an elevated or darker center. In about 79% of cases in Europe but only 19% of cases in endemic areas of the U.S., the rash gradually clears from the center toward the edges, possibly forming a "bull's eye" pattern. The rash may feel warm but usually is not itchy, is rarely tender or painful, and takes up to four weeks to resolve if untreated.
The EM (Erythema migrans) rash is often accompanied by symptoms of a viral-like illness, including fatigue, headache, body aches, fever, and chills, but usually not nausea or upper-respiratory problems. These symptoms may also appear without a rash, or linger after the rash disappears. Lyme can progress to later stages without these symptoms or a rash.
People with high fever for more than two days or whose other symptoms of viral-like illness do not improve despite antibiotic treatment for Lyme disease, or who have abnormally low levels of white or red cells or platelets in the blood, should be investigated for possible coinfection with other tick-borne diseases, such as ehrlichiosis and babesiosis.
Early disseminated infection
Within days to weeks after the onset of local infection, the Borrelia bacteria may spread through the lymphatic system or bloodstream. In 10-20% of untreated cases, EM rashes develop at sites across the body that bear no relation to the original tick bite. Transient muscle pains and joint pains are also common.
In about 10-15% of untreated people, Lyme causes neurological problems known as neuroborreliosis. Early neuroborreliosis typically appears 4–6 weeks (range 1–12 weeks) after the tick bite and involves some combination of lymphocytic meningitis, cranial neuritis, radiculopathy and/or mononeuritis multiplex. Lymphocytic meningitis causes characteristic changes in the cerebrospinal fluid (CSF) and may be accompanied for several weeks by variable headache and, less commonly, usually mild meningitis signs such as inability to flex the neck fully and intolerance to bright lights, but typically no or only very low fever. In children, partial loss of vision may also occur. Cranial neuritis is an inflammation of cranial nerves. When due to Lyme, it most typically causes facial palsy impairing blinking, smiling, and chewing in one or both sides of the face. It may also cause intermittent double vision. Lyme radiculopathy is an inflammation of spinal nerve roots that often causes pain and less often weakness, numbness, or altered sensation in the areas of the body served by nerves connected to the affected roots, e.g. limb(s) or part(s) of trunk. The pain is often described as unlike any other previously felt, excruciating, migrating, worse at night, rarely symmetrical, and often accompanied by extreme sleep disturbance. Mononeuritis multiplex is an inflammation causing similar symptoms in one or more unrelated peripheral nerves. Rarely, early neuroborreliosis may involve inflammation of the brain or spinal cord, with symptoms such as confusion, abnormal gait, ocular movements, or speech, impaired movement, impaired motor planning, or shaking.
In North America, facial palsy is the typical early neuroborreliosis presentation, occurring in 5-10% of untreated people, in about 75% of cases accompanied by lymphocytic meningitis. Lyme radiculopathy is reported half as frequently, but many cases may be unrecognized. In European adults, the most common presentation is a combination of lymphocytic meningitis and radiculopathy known as Bannwarth syndrome, accompanied in 36-89% of cases by facial palsy. In this syndrome, radicular pain tends to start in the same body region as the initial erythema migrans rash, if there was one, and precedes possible facial palsy and other impaired movement. In extreme cases, permanent impairment of motor or sensory function of the lower limbs may occur. In European children, the most common manifestations are facial palsy (in 55%), other cranial neuritis, and lymphocytic meningitis (in 27%).
In about 4-10% of untreated cases in the U.S. and 0.3-4% of untreated cases in Europe, typically between June and December, about one month (range 4 days-7 months) after the tick bite, the infection may cause heart complications known as Lyme carditis. Symptoms may include heart palpitations (in 69% of people), dizziness, fainting, shortness of breath, and chest pain. Other symptoms of Lyme disease may also be present, such as EM rash, joint aches, facial palsy, headaches, or radicular pain. In some people, however, carditis may be the first manifestation of Lyme disease. Lyme carditis in 19-87% of people adversely impacts the heart's electrical conduction system, causing atrioventricular block that often manifests as heart rhythms that alternate within minutes between abnormally slow and abnormally fast. In 10-15% of people, Lyme causes myocardial complications such as cardiomegaly, left ventricular dysfunction, or congestive heart failure.
Another skin condition, found in Europe but not in North America, is borrelial lymphocytoma, a purplish lump that develops on the ear lobe, nipple, or scrotum.
Late disseminated infection
After several months, untreated or inadequately treated people may go on to develop chronic symptoms that affect many parts of the body, including the joints, nerves, brain, eyes, and heart.
Lyme arthritis occurs in up to 60% of untreated people, typically starting about six months after infection. It usually affects only one or a few joints, often a knee or possibly the hip, other large joints, or the temporomandibular joint. There is usually large joint effusion and swelling, but only mild or moderate pain. Without treatment, swelling and pain typically resolve over time but periodically return. Baker's cysts may form and rupture. In some cases, joint erosion occurs.
Chronic neurologic symptoms occur in up to 5% of untreated people. A peripheral neuropathy or polyneuropathy may develop, causing abnormal sensations such as numbness, tingling or burning starting at the feet or hands and over time possibly moving up the limbs. A test may show reduced sensation of vibrations in the feet. An affected person may feel as if wearing a stocking or glove without actually doing so.
A neurologic syndrome called Lyme encephalopathy is associated with subtle memory and cognitive difficulties, insomnia, a general sense of feeling unwell, and changes in personality. However, problems such as depression and fibromyalgia are as common in people with Lyme disease as in the general population.
Lyme can cause a chronic encephalomyelitis that resembles multiple sclerosis. It may be progressive and can involve cognitive impairment, brain fog, migraines, balance issues, weakness in the legs, awkward gait, facial palsy, bladder problems, vertigo, and back pain. In rare cases, untreated Lyme disease may cause frank psychosis, which has been misdiagnosed as schizophrenia or bipolar disorder. Panic attacks and anxiety can occur; also, delusional behavior may be seen, including somatoform delusions, sometimes accompanied by a depersonalization or derealization syndrome, where the people begin to feel detached from themselves or from reality.
Acrodermatitis chronica atrophicans (ACA) is a chronic skin disorder observed primarily in Europe among the elderly. ACA begins as a reddish-blue patch of discolored skin, often on the backs of the hands or feet. The lesion slowly atrophies over several weeks or months, with the skin becoming first thin and wrinkled and then, if untreated, completely dry and hairless.
Cause



Lyme disease is caused by spirochetes, spiral bacteria from the genus Borrelia. Spirochetes are surrounded by peptidoglycan and flagella, along with an outer membrane similar to Gram-negative bacteria. Because of their double-membrane envelope, Borrelia bacteria are often mistakenly described as Gram negative despite the considerable differences in their envelope components from Gram-negative bacteria. The Lyme-related Borrelia species are collectively known as Borrelia burgdorferi sensu lato, and show a great deal of genetic diversity.
B. burgdorferi sensu lato is made up of 21 closely related species, but only four clearly cause Lyme disease: B. mayonii (found in North America), B. burgdorferi sensu stricto (predominant in North America, but also present in Europe), B. afzelii, and B. garinii (both predominant in Eurasia). Some studies have also proposed that B. bissettii and B. valaisiana may sometimes infect humans, but these species do not seem to be important causes of disease.
Transmission
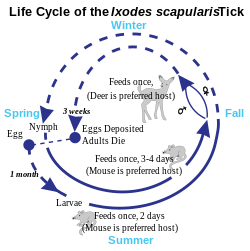
Lyme disease is classified as a zoonosis, as it is transmitted to humans from a natural reservoir among small mammals and birds by ticks that feed on both sets of hosts. Hard-bodied ticks of the genus Ixodes are the main vectors of Lyme disease (also the vector for Babesia). Most infections are caused by ticks in the nymphal stage, because they are very small and thus may feed for long periods of time undetected. Nymphal ticks are generally the size of a poppy seed and sometimes with a dark head and a translucent body. Or, the nymphal ticks can be darker. (The younger larval ticks are very rarely infected.) Although deer are the preferred hosts of adult deer ticks, and tick populations are much lower in the absence of deer, ticks generally do not acquire Borrelia from deer, instead they obtain them from infected small mammals such as the white-footed mouse, and occasionally birds. Areas where Lyme is common are expanding.
Within the tick midgut, the Borrelia's outer surface protein A (OspA) binds to the tick receptor for OspA, known as TROSPA. When the tick feeds, the Borrelia downregulates OspA and upregulates OspC, another surface protein. After the bacteria migrate from the midgut to the salivary glands, OspC binds to Salp15, a tick salivary protein that appears to have immunosuppressive effects that enhance infection. Successful infection of the mammalian host depends on bacterial expression of OspC.
Tick bites often go unnoticed because of the small size of the tick in its nymphal stage, as well as tick secretions that prevent the host from feeling any itch or pain from the bite. However, transmission is quite rare, with only about 1.2 to 1.4 percent of recognized tick bites resulting in Lyme disease.
In Europe, the vector is Ixodes ricinus, which is also called the sheep tick or castor bean tick. In China, Ixodes persulcatus (the taiga tick) is probably the most important vector. In North America, the black-legged tick or deer tick (Ixodes scapularis) is the main vector on the East Coast.
The lone star tick (Amblyomma americanum), which is found throughout the Southeastern United States as far west as Texas, is unlikely to transmit the Lyme disease spirochetes, though it may be implicated in a related syndrome called southern tick-associated rash illness, which resembles a mild form of Lyme disease.
On the West Coast of the United States, the main vector is the western black-legged tick (Ixodes pacificus). The tendency of this tick species to feed predominantly on host species such as the Western Fence Lizard that are resistant to Borrelia infection appears to diminish transmission of Lyme disease in the West.
Transmission can occur across the placenta during pregnancy and as with a number of other spirochetal diseases, adverse pregnancy outcomes are possible with untreated infection; prompt treatment with antibiotics reduces or eliminates this risk.
While Lyme spirochetes have been found in insects, as well as ticks, reports of actual infectious transmission appear to be rare. Lyme spirochete DNA has been found in semen and breast milk. However, according to the CDC, live spirochetes have not been found in breast milk, urine, or semen and thus is not sexually transmitted.
Tick-borne coinfections
Ticks that transmit B. burgdorferi to humans can also carry and transmit several other parasites, such as Theileria microti and Anaplasma phagocytophilum, which cause the diseases babesiosis and human granulocytic anaplasmosis (HGA), respectively. Among people with early Lyme disease, depending on their location, 2–12% will also have HGA and 2–40% will have babesiosis. Ticks in certain regions, including the lands along the eastern Baltic Sea, also transmit tick-borne encephalitis.
Coinfections complicate Lyme symptoms, especially diagnosis and treatment. It is possible for a tick to carry and transmit one of the coinfections and not Borrelia, making diagnosis difficult and often elusive. The Centers for Disease Control and Prevention studied 100 ticks in rural New Jersey, and found 55% of the ticks were infected with at least one of the pathogens.
Pathophysiology
B. burgdorferi can spread throughout the body during the course of the disease, and has been found in the skin, heart, joints, peripheral nervous system, and central nervous system. Many of the signs and symptoms of Lyme disease are a consequence of the immune response to spirochete in those tissues.
B. burgdorferi is injected into the skin by the bite of an infected Ixodes tick. Tick saliva, which accompanies the spirochete into the skin during the feeding process, contains substances that disrupt the immune response at the site of the bite. This provides a protective environment where the spirochete can establish infection. The spirochetes multiply and migrate outward within the dermis. The host inflammatory response to the bacteria in the skin causes the characteristic circular EM lesion. Neutrophils, however, which are necessary to eliminate the spirochetes from the skin, fail to appear in necessary numbers in the developing EM lesion, mostly due to the fact that tick saliva also inhibits neutrophil function. This allows the bacteria to survive and eventually spread throughout the body.
Days to weeks following the tick bite, the spirochetes spread via the bloodstream to joints, heart, nervous system, and distant skin sites, where their presence gives rise to the variety of symptoms of the disseminated disease. The spread of B. burgdorferi is aided by the attachment of the host protease plasmin to the surface of the spirochete.
If untreated, the bacteria may persist in the body for months or even years, despite the production of B. burgdorferi antibodies by the immune system. The spirochetes may avoid the immune response by decreasing expression of surface proteins that are targeted by antibodies, antigenic variation of the VlsE surface protein, inactivating key immune components such as complement, and hiding in the extracellular matrix, which may interfere with the function of immune factors.
In the brain, B. burgdorferi may induce astrocytes to undergo astrogliosis (proliferation followed by apoptosis), which may contribute to neurodysfunction. The spirochetes may also induce host cells to secrete quinolinic acid, which stimulates the NMDA receptor on nerve cells, which may account for the fatigue and malaise observed with Lyme encephalopathy. In addition, diffuse white matter pathology during Lyme encephalopathy may disrupt gray matter connections, and could account for deficits in attention, memory, visuospatial ability, complex cognition, and emotional status. White matter disease may have a greater potential for recovery than gray matter disease, perhaps because the neuronal loss is less common. Resolution of MRI white matter hyperintensities after antibiotic treatment has been observed.
Tryptophan, a precursor to serotonin, appears to be reduced within the central nervous system in a number of infectious diseases that affect the brain, including Lyme. Researchers are investigating if this neurohormone secretion is the cause of neuropsychiatric disorders developing in some people with borreliosis.
Immunological studies
Exposure to the Borrelia bacterium during Lyme disease possibly causes a long-lived and damaging inflammatory response, a form of pathogen-induced autoimmune disease. The production of this reaction might be due to a form of molecular mimicry, where Borrelia avoids being killed by the immune system by resembling normal parts of the body's tissues.
Chronic symptoms from an autoimmune reaction could explain why some symptoms persist even after the spirochetes have been eliminated from the body. This hypothesis may explain why chronic arthritis persists after antibiotic therapy, similar to rheumatic fever, but its wider application is controversial.
Diagnosis
Lyme disease is diagnosed based on symptoms, objective physical findings (such as erythema migrans (EM) rash, facial palsy, or arthritis), history of possible exposure to infected ticks, and possibly laboratory tests. People with symptoms of early Lyme disease should have a total body skin examination for EM rashes and asked whether EM-type rashes had manifested within the last 1–2 months. Presence of an EM rash and recent tick exposure (i.e., being outdoors in a likely tick habitat where Lyme is common, within 30 days of the appearance of the rash) are sufficient for Lyme diagnosis; no laboratory confirmation is needed or recommended. Most people who get infected do not remember a tick or a bite, and the EM rash need not look like a bull's eye (most EM rashes in the U.S. do not) or be accompanied by any other symptoms. In the U.S., Lyme is most common in the New England and Mid-Atlantic states and parts of Wisconsin and Minnesota, but it is expanding into other areas. Several bordering areas of Canada also have high Lyme risk.
In the absence of an EM rash or history of tick exposure, Lyme diagnosis depends on laboratory confirmation. The bacteria that cause Lyme disease are difficult to observe directly in body tissues and also difficult and too time-consuming to grow in the laboratory. The most widely used tests look instead for presence of antibodies against those bacteria in the blood. A positive antibody test result does not by itself prove active infection, but can confirm an infection that is suspected because of symptoms, objective findings, and history of tick exposure in a person. Because as many as 5-20% of the normal population have antibodies against Lyme, people without history and symptoms suggestive of Lyme disease should not be tested for Lyme antibodies: a positive result would likely be false, possibly causing unnecessary treatment.
In some cases, when history, signs, and symptoms are strongly suggestive of early disseminated Lyme disease, empiric treatment may be started and reevaluated as laboratory test results become available.
Laboratory testing
Tests for antibodies in the blood by ELISA and Western blot is the most widely used method for Lyme diagnosis. A two-tiered protocol is recommended by the Centers for Disease Control and Prevention (CDC): the sensitive ELISA test is performed first, and if it is positive or equivocal, then the more specific Western blot is run. The immune system takes some time to produce antibodies in quantity. After Lyme infection onset, antibodies of types IgM and IgG usually can first be detected respectively at 2–4 weeks and 4–6 weeks, and peak at 6–8 weeks. When an EM rash first appears, detectable antibodies may not be present. Therefore, it is recommended that testing not be performed and diagnosis be based on the presence of the EM rash. Up to 30 days after suspected Lyme infection onset, infection can be confirmed by detection of IgM or IgG antibodies; after that, it is recommended that only IgG antibodies be considered. A positive IgM and negative IgG test result after the first month of infection is generally indicative of a false positive result. The number of IgM antibodies usually collapses 4–6 months after infection, while IgG antibodies can remain detectable for years.
Other tests may be used in neuroborreliosis cases. In Europe, neuroborreliosis is usually caused by Borrelia garinii and almost always involves lymphocytic pleocytosis, i.e. the densities of lymphocytes (infection-fighting cells) and protein in the cerebrospinal fluid (CSF) typically rise to characteristically abnormal levels, while glucose level remains normal. Additionally, the immune system produces antibodies against Lyme inside the intrathecal space, which contains the CSF. Demonstration by lumbar puncture and CSF analysis of pleocytosis and intrathecal antibody production are required for definite diagnosis of neuroborreliosis in Europe (except in cases of peripheral neuropathy associated with acrodermatitis chronica atrophicans, which usually is caused by Borrelia afzelii and confirmed by blood antibody tests). In North America, neuroborreliosis is caused by Borrelia burgdorferi and may not be accompanied by the same CSF signs; they confirm a diagnosis of central nervous system (CNS) neuroborreliosis if positive, but do not exclude it if negative. American guidelines consider CSF analysis optional when symptoms appear to be confined to the peripheral nervous system (PNS), e.g. facial palsy without overt meninigitis symptoms. Unlike blood and intrathecal antibody tests, CSF pleocytosis tests revert to normal after infection ends and therefore can be used as objective markers of treatment success and inform decisions on whether to retreat. In infection involving the PNS, electromyography and nerve conduction studies can be used to monitor objectively the response to treatment.
In Lyme carditis, electrocardiograms are used to evidence heart conduction abnormalities, while echocardiography may show myocardial dysfunction. Biopsy and confirmation of Borrelia cells in myocardial tissue may be used in specific cases but are usually not done because of risk of the procedure.
Polymerase chain reaction (PCR) tests for Lyme disease have also been developed to detect the genetic material (DNA) of the Lyme disease spirochete. Culture or PCR are the current means for detecting the presence of the organism, as serologic studies only test for antibodies of Borrelia. PCR has the advantage of being much faster than culture. However, PCR tests are susceptible to false positive results, e.g. by detection of debris of dead Borrelia cells or specimen contamination. Even when properly performed, PCR often shows false negative results because few Borrelia cells can be found in blood and cerebrospinal fluid (CSF) during infection. Hence, PCR tests are recommended only in special cases, e.g. diagnosis of Lyme arthritis, because it is a highly sensitive way of detecting ospA DNA in synovial fluid. Although sensitivity of PCR in CSF is low, its use may be considered when intrathecal antibody production test results are suspected of being falsely negative, e.g. in very early (< 6 weeks) neuroborreliosis or in immunosuppressed people.
Several other forms of laboratory testing for Lyme disease are available, some of which have not been adequately validated. OspA antigens, shed by live Borrelia bacteria into urine, are a promising technique being studied. The use of nanotrap particles for their detection is being looked at and the OspA has been linked to active symptoms of Lyme. High titers of either immunoglobulin G (IgG) or immunoglobulin M (IgM) antibodies to Borrelia antigens indicate disease, but lower titers can be misleading, because the IgM antibodies may remain after the initial infection, and IgG antibodies may remain for years.
The CDC does not recommend urine antigen tests, PCR tests on urine, immunofluorescent staining for cell-wall-deficient forms of B. burgdorferi, and lymphocyte transformation tests.
Imaging
Neuroimaging is controversial in whether it provides specific patterns unique to neuroborreliosis, but may aid in differential diagnosis and in understanding the pathophysiology of the disease. Though controversial, some evidence shows certain neuroimaging tests can provide data that are helpful in the diagnosis of a person. Magnetic resonance imaging (MRI) and single-photon emission computed tomography (SPECT) are two of the tests that can identify abnormalities in the brain of a person affected with this disease. Neuroimaging findings in an MRI include lesions in the periventricular white matter, as well as enlarged ventricles and cortical atrophy. The findings are considered somewhat unexceptional because the lesions have been found to be reversible following antibiotic treatment. Images produced using SPECT show numerous areas where an insufficient amount of blood is being delivered to the cortex and subcortical white matter. However, SPECT images are known to be nonspecific because they show a heterogeneous pattern in the imaging. The abnormalities seen in the SPECT images are very similar to those seen in people with cerebral vacuities and Creutzfeldt–Jakob disease, which makes them questionable.
Differential diagnosis
Community clinics have been reported to misdiagnose 23–28% of Erythema migrans (EM) rashes and 83% of other objective manifestations of early Lyme disease. EM rashes are often misdiagnosed as spider bites, cellulitis, or shingles. Many misdiagnoses are credited to the widespread misconception that EM rashes should look like a bull's eye. Actually, the key distinguishing features of the EM rash are the speed and extent to which it expands, respectively up to 2–3 cm/day and a diameter of at least 5 cm, and in 50% of cases more than 16 cm. The rash expands away from its center, which may or may not look different or be separated by ring-like clearing from the rest of the rash. Compared to EM rashes, spider bites are more common in the limbs, tend to be more painful and itchy or become swollen, and some may cause necrosis (sinking dark blue patch of dead skin). Cellulitis most commonly develops around a wound or ulcer, is rarely circular, and is more likely to become swollen and tender. EM rashes often appear at sites that are unusual for cellulitis, such as the armpit, groin, abdomen, or back of knee. Like Lyme, shingles often begins with headache, fever, and fatigue, which are followed by pain or numbness. However, unlike Lyme, in shingles these symptoms are usually followed by appearance of rashes composed of multiple small blisters along a nerve's dermatome, and shingles can also be confirmed by quick laboratory tests.
Facial palsy caused by Lyme disease (LDFP) is often misdiagnosed as Bell's palsy. Although Bell's palsy is the most common type of one-sided facial palsy (about 70% of cases), LDFP can account for about 25% of cases of facial palsy in areas where Lyme disease is common. Compared to LDFP, Bell's palsy much less frequently affects both sides of the face. Even though LDFP and Bell's palsy have similar symptoms and evolve similarly if untreated, corticosteroid treatment is beneficial for Bell's Palsy, while being detrimental for LDFP. Recent history of exposure to a likely tick habitat during warmer months, EM rash, viral-like symptoms such as headache and fever, and/or palsy in both sides of the face should be evaluated for likelihood of LDFP; if it is more than minimal, empiric therapy with antibiotics should be initiated, without corticosteroids, and reevaluated upon completion of laboratory tests for Lyme disease.
Unlike viral meningitis, Lyme lymphocytic meningitis tends to not cause fever, last longer, and recur. Lymphocytic meningitis is also characterized by possibly co-occurring with EM rash, facial palsy, or partial vision obstruction and having much lower percentage of polymorphonuclear leukocytes in CSF.
Lyme radiculopathy affecting the limbs is often misdiagnosed as a radiculopathy caused by nerve root compression, such as sciatica. Although most cases of radiculopathy are compressive and resolve with conservative treatment (e.g., rest) within 4–6 weeks, guidelines for managing radiculopathy recommend first evaluating risks of other possible causes that, although less frequent, require immediate diagnosis and treatment, including infections such as Lyme and shingles. A history of outdoor activities in likely tick habitats in the last 3 months possibly followed by a rash or viral-like symptoms, and current headache, other symptoms of lymphocytic meningitis, or facial palsy would lead to suspicion of Lyme disease and recommendation of serological and lumbar puncture tests for confirmation.
Lyme radiculopathy affecting the trunk can be misdiagnosed as myriad other conditions, such as diverticulitis and acute coronary syndrome. Diagnosis of late-stage Lyme disease is often complicated by a multifaceted appearance and nonspecific symptoms, prompting one reviewer to call Lyme the new "great imitator". Lyme disease may be misdiagnosed as multiple sclerosis, rheumatoid arthritis, fibromyalgia, chronic fatigue syndrome, lupus, Crohn's disease, HIV, or other autoimmune and neurodegenerative diseases. As all people with later-stage infection will have a positive antibody test, simple blood tests can exclude Lyme disease as a possible cause of a person's symptoms.
Prevention
Tick bites may be prevented by avoiding or reducing time in likely tick habitats and taking precautions while in and when getting out of one.
Most Lyme human infections are caused by Ixodes nymph bites between April and September. Ticks prefer moist, shaded locations in woodlands, shrubs, tall grasses and leaf litter or wood piles. Tick densities tend to be highest in woodlands, followed by unmaintained edges between woods and lawns (about half as high), ornamental plants and perennial groundcover (about a quarter), and lawns (about 30 times less). Ixodes larvae and nymphs tend to be abundant also where mice nest, such as stone walls and wood logs. Ixodes larvae and nymphs typically wait for potential hosts ("quest") on leaves or grasses close to the ground with forelegs outstretched; when a host brushes against its limbs, the tick rapidly clings and climbs on the host looking for a skin location to bite. In Northeastern United States, 69% of tick bites are estimated to happen in residences, 11% in schools or camps, 9% in parks or recreational areas, 4% at work, 3% while hunting, and 4% in other areas. Activities associated with tick bites around residences include yard work, brush clearing, gardening, playing in the yard, and letting into the house dogs or cats that roam outside in woody or grassy areas. In parks, tick bites often happen while hiking or camping. Walking on a mowed lawn or center of a trail without touching adjacent vegetation is less risky than crawling or sitting on a log or stone wall. Pets should not be allowed to roam freely in likely tick habitats.
As a precaution, CDC recommends soaking or spraying clothes, shoes, and camping gear such as tents, backpacks and sleeping bags with 0.5% permethrin solution and hanging them to dry before use. Permethrin is odorless and safe for humans but highly toxic to ticks. After crawling on permethrin-treated fabric for as few as 10–20 seconds, tick nymphs become irritated and fall off or die. Permethrin-treated closed-toed shoes and socks reduce by 74 times the number of bites from nymphs that make first contact with a shoe of a person also wearing treated shorts (because nymphs usually quest near the ground, this is a typical contact scenario). Better protection can be achieved by tucking permethrin-treated trousers (pants) into treated socks and a treated long-sleeve shirt into the trousers so as to minimize gaps through which a tick might reach the wearer's skin. Light-colored clothing may make it easier to see ticks and remove them before they bite. Military and outdoor workers' uniforms treated with permethrin have been found to reduce the number of bite cases by 80-95%. Permethrin protection lasts several weeks of wear and washings in customer-treated items and up to 70 washings for factory-treated items. Permethrin should not be used on human skin, underwear or cats.
The EPA recommends several tick repellents for use on exposed skin, including DEET, picaridin, IR3535 (a derivative of amino acid beta-alanine), oil of lemon eucalyptus (OLE, a natural compound) and OLE's active ingredient para-menthane-diol (PMD). Unlike permethrin, repellents repel but do not kill ticks, protect for only several hours after application, and may be washed off by sweat or water. The most popular repellent is DEET in the U.S. and picaridin in Europe. Unlike DEET, picaridin is odorless and is less likely to irritate the skin or harm fabric or plastics. Repellents with higher concentration may last longer but are not more effective; against ticks, 20% picaridin may work for 8 hours vs. 55–98.11% DEET for 5–6 hours or 30-40% OLE for 6 hours. Repellents should not be used under clothes, on eyes, mouth, wounds or cuts, or on babies younger than 2 months (3 years for OLE or PMD). If sunscreen is used, repellent should be applied on top of it. Repellents should not be sprayed directly on a face, but should instead be sprayed on a hand and then rubbed on the face.
After coming indoors, clothes, gear and pets should be checked for ticks. Clothes can be put into a hot dryer for 10 minutes to kill ticks (just washing or warm dryer are not enough). Showering as soon as possible, looking for ticks over the entire body, and removing them reduce risk of infection. Unfed tick nymphs are the size of a poppy seed, but a day or two after biting and attaching themselves to a person, they look like a small blood blister. The following areas should be checked especially carefully: armpits, between legs, back of knee, bellybutton, trunk, and in children ears, neck and hair.
Tick removal

Attached ticks should be removed promptly. Risk of infection increases with time of attachment, but in North America risk of Lyme disease is small if the tick is removed within 36 hours. CDC recommends inserting a fine-tipped tweezer between the skin and the tick, grasping very firmly, and pulling the closed tweezer straight away from the skin without twisting, jerking, squeezing or crushing the tick. After tick removal, any tick parts remaining in the skin should be removed with the tweezer, if possible. Wound and hands should then be cleaned with alcohol or soap and water. The tick may be disposed by placing it in a container with alcohol, sealed bag, tape or flushed down the toilet. The bitten person should write down where and when the bite happened so that this can be informed to a doctor if the person gets a rash or flu-like symptoms in the following several weeks. CDC recommends not using fingers, nail polish, petroleum jelly or heat on the tick to try to remove it.
In Australia, where the Australian paralysis tick is prevalent, the Australasian Society of Clinical Immunology and Allergy recommends not using tweezers to remove ticks, because if the person is allergic, anaphylaxis could result. Instead, a product should be sprayed on the tick to cause it to freeze and then drop off. A doctor would use liquid nitrogen, but products available from chemists for freezing warts can be used instead. Another method originating from Australia consists in using about 20 cm of dental floss or fishing line for slowly tying an overhand knot between the skin and the tick and then pulling it away from the skin.
Preventive antibiotics
The risk of infectious transmission increases with the duration of tick attachment. It requires between 36 and 48 hours of attachment for the bacteria that causes Lyme to travel from within the tick into its saliva. If a deer tick that is sufficiently likely to be carrying Borrelia is found attached to a person and removed, and if the tick has been attached for 36 hours or is engorged, a single dose of doxycycline administered within the 72 hours after removal may reduce the risk of Lyme disease. It is not generally recommended for all people bitten, as development of infection is rare: about 50 bitten people would have to be treated this way to prevent one case of erythema migrans (i.e. the typical rash found in about 70–80% of people infected).
Garden landscaping
Several landscaping practices may reduce risk of tick bites in residential yards. The lawn should be kept mowed, leaf litter and weeds removed and groundcover use avoided. Woodlands, shrubs, stone walls and wood piles should be separated from the lawn by a 3-ft-wide rock or woodchip barrier. Without vegetation on the barrier, ticks will tend not to cross it; acaricides may also be sprayed on it to kill ticks. A sun-exposed tick-safe zone at least 9 ft from the barrier should concentrate human activity on the yard, including any patios, playgrounds and gardening. Materials such as wood decking, concrete, bricks, gravel or woodchips may be used on the ground under patios and playgrounds so as to discourage ticks there. An 8-ft-high fence may be added to keep deer away from the tick-safe zone.
Occupational exposure
Outdoor workers are at risk of Lyme disease if they work at sites with infected ticks. This includes construction, landscaping, forestry, brush clearing, land surveying, farming, railroad work, oil field work, utility line work, park or wildlife management. U.S. workers in the northeastern and north-central states are at highest risk of exposure to infected ticks. Ticks may also transmit other tick-borne diseases to workers in these and other regions of the country. Worksites with woods, bushes, high grass or leaf litter are likely to have more ticks. Outdoor workers should be most careful to protect themselves in the late spring and summer when young ticks are most active.
Host animals
Lyme and other deer tick-borne diseases can sometimes be reduced by greatly reducing the deer population on which the adult ticks depend for feeding and reproduction. Lyme disease cases fell following deer eradication on an island, Monhegan, Maine, and following deer control in Mumford Cove, Connecticut. It is worth noting that eliminating deer may lead to a temporary increase in tick density.
For example, in the U.S., reducing the deer population to levels of 8 to 10 per square mile (from the current levels of 60 or more deer per square mile in the areas of the country with the highest Lyme disease rates) may reduce tick numbers and reduce the spread of Lyme and other tick-borne diseases. However, such a drastic reduction may be very difficult to implement in many areas, and low to moderate densities of deer or other large mammal hosts may continue to feed sufficient adult ticks to maintain larval densities at high levels. Routine veterinary control of ticks of domestic animals, including livestock, by use of acaricides can contribute to reducing exposure of humans to ticks.
In Europe, known reservoirs of Borrelia burgdorferi were 9 small mammals, 7 medium-sized mammals and 16 species of birds (including passerines, sea-birds and pheasants). These animals seem to transmit spirochetes to ticks and thus participate in the natural circulation of B. burgdorferi in Europe. The house mouse is also suspected