Neurofibromatosis, Type I
A number sign (#) is used with this entry because neurofibromatosis type I (NF1) is caused by heterozygous mutation in the neurofibromin gene (NF1; 613113) on chromosome 17q11.
DescriptionNeurofibromatosis type I is an autosomal dominant disorder characterized by cafe-au-lait spots, Lisch nodules in the eye, and fibromatous tumors of the skin. Individuals with the disorder have increased susceptibility to the development of benign and malignant tumors. NF1 is sometimes referred to as 'peripheral neurofibromatosis.' The worldwide incidence of NF1 is 1 in 2,500 to 1 in 3,000 individuals (reviews by Shen et al., 1996 and Williams et al., 2009).
Type II neurofibromatosis (NF2; 101000) is a genetically distinct disorder caused by mutation in the gene encoding merlin (NF2; 607379) on chromosome 22q12. NF2, sometimes known as 'central neurofibromatosis,' is characterized by bilateral acoustic neuroma and meningioma, but few skin lesions or neurofibromas (Rouleau et al., 1993).
Some patients with homozygous or compound heterozygous mutations in mismatch repair genes (see, e.g., MLH1; 120436 and MSH2; 609309) have a phenotype characterized by early onset malignancies and mild features of NF1, especially cafe-au-lait spots; this is known as the mismatch repair cancer syndrome (276300), sometimes referred to as brain tumor-polyposis syndrome-1 or Turcot syndrome. These patients typically do not have germline mutations in the NF1 gene, although a study by Wang et al. (2003) suggested that biallelic mutations in mismatch repair genes may cause somatic mutations in the NF1 gene, perhaps resulting in isolated features resembling NF1.
See also Legius syndrome (611431), a genetically distinct disorder with a similar phenotype to NF1.
Clinical FeaturesSorensen et al. (1986) conducted a valuable follow-up study of the natural history of NF1 in a nationwide cohort of 212 patients with the disorder identified in Denmark by Borberg (1951). Malignant neoplasms or benign CNS tumors occurred in 45% of the probands, giving a relative risk of 4.0 compared with expected numbers. All 76 probands had been ascertained through hospitals and were more severely affected than their incidentally identified relatives, although relatives had poorer survival rates than persons in the general population. The worst prognosis was shown by female probands.
Friedman et al. (1993) described a central database designed to collect information on NF1 from 16 centers around the world. The aspects of the disorder for which information was being collected included renal artery stenosis and cerebral artery stenosis.
Dugoff and Sujansky (1996) reported outcome data of 247 pregnancies in 105 women with NF1. The 247 pregnancies resulted in 44 first trimester spontaneous abortions. The cesarean section rate (36%) was greater than in the general population (9.1 to 23.5%). In 7 of the patients, cesarean section was required because of maternal complications of NF1 including pelvic neurofibromas, pelvic bony abnormality with or without kyphoscoliosis, pheochromocytoma, and spinal cord neurofibroma. Dugoff and Sujansky (1996) reported that 80% of the women in their study experienced either the appearance of new neurofibromas, growth of existing neurofibromas, or both. Thirty-three percent of these women noted a decrease in the size of their neurofibromas in the postpartum period. Eighteen percent of the women reported no changes in neurofibromas and no appearance of new neurofibromas during pregnancy.
Friedman and Birch (1997) summarized clinical information about NF1 patients based on the International Database maintained by the National NF Foundation (NNFF), which contained information on 1,479 probands and 249 of their affected relatives with NF1 at the time of analysis. The age at diagnosis of NF1 was 8 years younger in the probands than in the affected relatives, and many of the manifestations of NF1 were more frequent in the probands than in their affected relatives. The age-specific prevalence of most manifestations of NF1 increased with age. Despite biases inherent in a convenience sample from specialist clinics, the frequency of manifestations of NF1 in many of the series was similar to those in 2 smaller population-based studies. Lisch nodules were said to be present in 57% of probands and 69.9% of affected relatives.
McGaughran et al. (1999) reported a study of 523 individuals from 304 families with NF1. More than 6 cafe-au-lait patches were seen in 383 of 442 (86.7%); 310 of 370 (83.8%) had axillary freckling; 151 of 357 (42.3%) had inguinal freckling; and 157 of 249 (63%) had Lisch nodules. Cutaneous neurofibromas were seen in 217 of 365 (59.4%), and subcutaneous tumors were present in 150 of 330 (45.5%). A positive family history of NF1 was found in 327 of 459 (71.2%). Learning disabilities of varying severity were seen in 186 of 300 (62%), and 49 (9.4%) of patients had CNS tumors, 25 of which were optic gliomas. Scoliosis was seen in 11.7%; 1.9% had pseudoarthrosis; 4.3% had epilepsy; and 2.1% had spinal neurofibromas.
Macrocephaly and short stature have been reported in several clinical studies of NF1. Clementi et al. (1999) studied growth in 528 NF1 patients obtained from a population-based registry in northeast Italy. Although macrocephaly was a consistent and common finding in NF1, short stature was less prominent and less frequent than previously reported. No differences in height were apparent between NF1 and normal subjects up to 7 years of age in girls and 12 years of age in boys. Clementi et al. (1999) presented growth charts for use by physicians following NF1 patients to assist in the identification of the effects of secondary growth disorders, for growth prognosis, and for evaluation of the effects of therapy.
Szudek et al. (2000) presented growth charts derived from study of 569 white North American children with NF1. They found that stature and occipitofrontal circumference (OFC) measurements were shifted and unimodal, with 13% of children being at or more than 2 SD below mean and 24% having OFC at or more than 2 SD above mean.
Rasmussen et al. (2001) used Multiple-Cause Mortality Files, compiled from U.S. death certificates by the National Center for Health Statistics for 1983-1997, to obtain information on mortality in NF1. They identified 3,770 cases among 32,722,122 deaths in the United States, a frequency of 1 in 8,700, which is one-third to one-half the estimated prevalence. Mean and median ages at death for persons with NF1 were 54.4 and 59 years, respectively, compared with 70.1 and 74 years in the general population. Results of proportionate mortality ratio (PMR) analyses showed that persons with NF1 were 34 times more likely to have a malignant connective or other soft-tissue neoplasm listed on their death certificates than were persons without NF1. Overall, persons with NF1 were 1.2 times more likely than expected to have a malignant neoplasm listed on their death certificates, but the PMR was 6.07 for persons who died at 10 to 19 years of age and was 4.93 for those who died at 20 to 29 years of age. Similarly, vascular disease was recorded more often than expected on death certificates of persons with NF1 who died before 30 years of age, but not in older persons.
Szudek et al. (2003) studied statistical associations among 13 of the most common or significant clinical features of NF1 in data from 4 large sets of NF1 patients comprising about 3,000 patients. The results suggested grouping 9 of the clinical features into 3 sets: (1) cafe-au-lait spots, intertriginous freckling, and Lisch nodules; (2) cutaneous, subcutaneous, and plexiform neurofibromas; (3) macrocephaly, optic glioma, other neoplasms. In addition, 3-way interactions among cafe-au-lait spots, intertriginous freckling, and subcutaneous neurofibromas indicated that the first 2 groups are not independent. Cafe-au-lait spots, intertriginous freckles, and Lisch nodules are all derived from cells of melanocytic origin, which derive from the embryonic neural crest. Thus, NF1 can be considered a neurocristopathy. The common thread between optic gliomas, other neoplasms, and macrocephaly may be glial hyperplasia. There was an observed association between pseudarthrosis and other neoplasms, which was more difficult to understand. Szudek et al. (2003) noted that these results cannot be used to predict which NF1 patients will get which particular features, but suggest that some affected individuals may be more likely than others to develop certain features of the disease.
Khosrotehrani et al. (2003) performed a cohort study among 378 NF1 patients receiving more than 1 year of follow-up care at an NF1 referral center in France. Clinical features, especially dermatologic, were evaluated as potential factors associated with mortality. Factors associated independently with mortality were the presence of subcutaneous neurofibromas (odds ratio, 10.8; 95% CI, 2.1-56.7; p less than 0.001), the absence of cutaneous neurofibromas (odds ratio, 5.3; 95% CI, 1.2-25.0; p = 0.03), and facial asymmetry (odds ratio, 11.4; 95% CI, 2.6-50.2; p less than 0.01). The absence of cutaneous neurofibromas in adulthood associated with high mortality may correspond to a subtype of NF1, familial spinal neurofibromatosis (162210). Khosrotehrani et al. (2003) concluded that features that can be found by a routine clinical examination are associated with mortality in patients with NF1, and that clinical follow-up should be focused on patients with subcutaneous neurofibromas, absence of cutaneous neurofibromas, and/or facial asymmetry. In a parallel study of a cohort of 703 NF1 patients in North America, Khosrotehrani et al. (2005) validated the observation that subcutaneous neurofibromas were associated with mortality.
Skin Manifestations
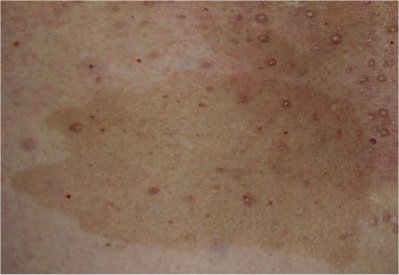
Variable numbers of hyperpigmented cafe-au-lait spots usually develop in the first years of life, but may be present at birth, and are often the first apparent feature of NF1. The quantity and size of these macules has not been linked to disease severity, and they show no tendency to malignant degeneration. The presence of 6 or more cafe-au-lait macules with diameter 0.5 cm before puberty or 1.5 cm after puberty is a diagnostic feature (see DIAGNOSIS below). Axillary and inguinal freckling ('Crowe sign') are usually noted between 3 and 5 years of age. Freckling can also occur above the eyelids, around the neck, and under the breasts. (reviews by Ferner et al., 2007 and Williams et al., 2009).
Neurofibromas are benign Schwann cell tumors that are classified according to their appearance and location: focal or diffuse cutaneous, subcutaneous, nodular or diffuse plexiform, and spinal. Focal cutaneous or dermal neurofibromas typically appear in late childhood or early adolescence, rarely cause pain or neurologic deficits, and do not transform into malignant tumors. Subcutaneous lesions can be noted on palpation of the skin and may present with tenderness or tingling distributed along the affected nerve. Plexiform neurofibromas arise from nerve fascicles, tend to grow along the length of the nerve, may involve multiple nerve branches and plexuses, and can cause significant morbidity. The growth rate is unpredictable, and soft tissue hypertrophy is often noted. Only the plexiform type of neurofibromas have a potential for transformation into malignant peripheral nerve sheath tumors (MPNST, see below) (reviews by Rosser and Packer, 2002; Ferner et al., 2007, and Williams et al., 2009).
Waggoner et al. (2000) conducted a retrospective review of neurofibromas among NF1 patients seen in a tertiary care referral center. Sixty-eight (16.8%) of 405 patients with NF1 had plexiform neurofibromas, which were located on the trunk (43%), the head and neck region (42%), and the extremities (15%). About 44% of these tumors were detected by 5 years of age. Presenting symptoms were most often related to the increasing size of the tumor, a loss of function (usually weakness), or pain. Only 2 patients (3%) developed malignant peripheral nerve sheath tumors in their preexisting plexiform neurofibromas. No specific NF1 features were associated with plexiform tumors.
To analyze growth rate and prognostic factors for progression of postoperative plexiform neurofibromas in patients with NF1, Nguyen et al. (2013) studied 52 patients (mean age 25 years, range 3-64 years) with 56 plexiform neurofibromas and looked at postoperative tumor volume change per year on MRI. Initial median tumor volume was 40.3 mL. Surgical indications included disfigurement in 21 patients, pain in 20 patients, and functional deficits in 16 patients. Sixteen percent of all cases experienced acute surgical complication, and 13% showed late complication. Eight patients (19%; 6 children, 2 adults) with residual tumor had repeat surgery for tumor progression. Median tumor progression was 0.6% change per year and 2.9% from baseline. Patients aged 21 years and younger had the highest progression rate (p less than 0.01). For every year of age the mean growth rate decreased by -0.463 mean percent (p = 0.03). With age as a continuous variable, age, the site of the tumor, and depth were the only factors associated with tumor progression. Fourteen plexiform neurofibromas (10 nodular and 4 diffuse) in 13 patients (5 children and 8 adults) were completely resected by visualization and did not relapse during observation (mean: 2.9 years; range: 1.1-5.8 years). Nguyen et al. (2013) concluded that age, tumor type, location, and depth are helpful to estimate the progression of plexiform neurofibromas after surgery and that patients benefit from elective surgery of small and completely removable plexiform neurofibromas.
Ophthalmologic Manifestations
Williams et al. (2009) noted that Lisch nodules, melanocytic iris hamartomas that do not affect vision, are pathognomonic of NF1.
Perry and Font (1982) used electron microscopic studies to demonstrate that the spindle-shaped cells within Lisch nodules are of melanocytic origin and represent melanocytic hamartomas. Thus, Lisch nodules are true tumors, not merely hyperpigmented patches.
Zehavi et al. (1986) found Lisch nodules in 73% of 30 NF1 cases, and found that their presence correlated directly with the severity of skin manifestations. Lisch nodules appeared as smooth, well-defined, gelatinous masses protruding from the surface of the iris on slit-lamp examination.
Ragge et al. (1993) provided a comprehensive discussion of Lisch nodules accompanied by colored photographs in irides of different colors. They pointed out that iris nodules were reported by several workers in the decade before the paper by Lisch (1937). In particular, Sakurai (1935) published a beautifully illustrated paper linking characteristic iris nodules with von Recklinghausen neurofibromatosis. Ragge et al. (1993) suggested that the lesions be renamed Sakurai-Lisch nodules in her honor.
On rare occasions, fibromas may occur in the iris, and glaucoma may occur (Grant and Walton, 1968). Westerhof et al. (1983) found hypertelorism in 24% of patients with neurofibromatosis.
Yasunari et al. (2000) studied 33 eyes of 17 consecutive NF1 patients diagnosed with NF1 by conventional ophthalmoscopy and by noninvasive infrared monochromatic light with confocal scanning laser ophthalmoscopy (SLO). Twenty-one digital fluorescein and indocyanine-green angiographies were obtained from 11 adult patients, and 77 angiograms were obtained from age-matched controls. Infrared monochromatic light examination by confocal SLO showed multiple bright patchy regions at and around the entire posterior pole of all 33 NF1 eyes. All bright patchy regions seen in adult patients corresponded to hypofluorescent areas on their indocyanine-green angiograms; however, no abnormalities were noted in any patient at corresponding areas under conventional ophthalmoscopic examination or fluorescein angiography. Control patients and their angiograms showed no choroidal abnormalities. Iris nodules were noted in 25 eyes (76%) of 14 patients (82%) and eyelid neurofibroma in 5 patients (29%). Since choroidal abnormalities were detected in 100% of NF1 patients examined, Yasunari et al. (2000) suggested that this abnormality be included in the diagnostic criteria for NF1.
Otsuka et al. (2001) performed serial ophthalmologic exams on 70 patients of various ages with NF1. Lisch nodules were found in 80% of patients of all ages and in two-thirds of patients younger than 10 years. Only 2 of 45 individuals older than age 10 years did not have Lisch nodules. Lisch nodules were more frequent in familial cases than in sporadic cases. Cutaneous neurofibromas developed at the average +/- SD age of 15.1 +/- 3.6 years in patients who had more than 10 Lisch nodules and at 21.8 +/- 3.9 years in those who had fewer than 10 Lisch nodules. The former group was significantly younger than the latter.
Lee et al. (2004) classified the periorbital deformities of adult orbitotemporal NF, reported previously undescribed clinical findings, and recommended guidelines for surgical treatment as well as management of surgical complications. They proposed a new classification for periorbital deformities: (1) brow ptosis; (2) upper eyelid infiltration with ptosis; (3) lower eyelid infiltration; (4) lateral canthal disinsertion; and (5) conjunctival and lacrimal gland infiltration. Of 33 patients over age 16 years with orbitotemporal NF, 2 (6%) had bilateral involvement whereas 31 (94%) had unilateral orbitotemporal NF. Previously undescribed findings included severe brow infiltration, lacrimal gland involvement, and functional nasolacrimal duct obstruction.
Optic Pathway Gliomas
Optic pathway gliomas (OPGs) are typically low-grade pilocytic astrocytomas that involve some combination of the optic nerves, chiasm, or optic tracts that occur in about 15% of children with NF1. OPGs are the most common intracranial malignancy in NF1. While most have a benign course, some may manifest as precocious puberty (reviews by Ferner et al., 2007 and Williams et al., 2009).
A longitudinal study of 219 patients with NF1 reported that clinical precocious puberty developed in 7 children, all of whom had optic chiasmal tumors (Listernick et al. (1994, 1995)).
Parazzini et al. (1995) documented spontaneous regression of optic pathway lesions in 4 NF1 patients and cautioned against diagnosis of optic nerve glioma without evidence of progression.
Parsa et al. (2001) observed spontaneous regression of large, clinically symptomatic optic gliomas in 13 patients, 5 with and 8 without NF1. Regression manifested as an overall shrinkage in tumor size or as a signal change on serial MRI. A variable degree of improvement in visual function accompanied regression. The authors concluded that the possibility of spontaneous regression of an optic glioma should be considered in planning the treatment of patients with these tumors.
Balcer et al. (2001) examined the neuroophthalmologic records and brain/orbital MRI scans from 43 consecutive pediatric NF1 patients with optic pathway gliomas. Involvement of the optic tracts and other postchiasmal structures was associated with a significantly higher probability of visual acuity loss. Visual loss was noted in 47% of patients at a median age of 4 years. However, 7% of patients developed initial visual loss during adolescence. The authors recommended close follow-up beyond the early childhood years, particularly for those children with postchiasmal tumor.
Singhal et al. (2002) compared the natural history of sporadic and NF1-associated optic gliomas in a series of 52 patients from northwest Britain. Ages at presentation were similar, but those associated with NF1 were less likely to present with impaired vision. Although NF1 optic gliomas were less aggressive, there was little difference in 5- and 10-year mortality rates between the 2 tumor groups. NF1 optic glioma cases were also at risk of a second primary central nervous system tumor; in 2 of 5 cases this occurred following radiotherapy, suggesting an etiologic link.
Thiagalingam et al. (2004) reviewed the natural history of optic pathway gliomas in 54 patients with NF1. The mean age at the time of diagnosis was 5.2 years, with 32 patients having signs or symptoms at the time of diagnosis. Seventeen patients were diagnosed after the age of 6 years. Twenty-two patients had tumor progression within 1 year of diagnosis and 6 patients showed progression after 1 year. Most conditions were managed conservatively (68.5%). At follow-up, 17 patients (31.5%) had severe visual impairment in their worse eye and 16.7% had bilateral moderate to severe visual impairment. Contrary to previous reports (e.g., Balcer et al., 2001), these results showed that optic pathway gliomas in patients with NF1 often presented in older children and might progress some time after diagnosis. Given the potential for serious visual consequences, the authors stressed the need for regular ophthalmologic monitoring of patients with NF1 for a long duration.
Liu et al. (2004) described the clinical and radiologic features of 7 children with NF1 with optic pathway gliomas involving the pregeniculate optic pathway in addition to the optic radiations. Two of the patients had expanding mass lesions within the white matter of the temporal or parietal lobes, which were histopathologically demonstrated to be pilocytic astrocytomas; the other 5 had radiographic involvement of the optic radiations but did not undergo biopsy. In 3 of the cases, the visual acuity was 20/200 or worse in each eye. Liu et al. (2004) concluded that optic pathway gliomas in NF1 rarely involve the optic radiations, but that optic radiation involvement might signal a more aggressive optic pathway glioma in patients with NF1.
Malignant Peripheral Nerve Sheath Tumors
One of the most clinically aggressive cancers associated with NF1 is the malignant peripheral nerve sheath tumor (MPNST), estimated to occur in 3 to 15% of patients over a lifetime (Knight et al., 1973).
King et al. (2000) reviewed 1,475 individuals with NF1 from a cohort of patients examined by a single investigator, Vincent M. Riccardi, between 1977 and 1996. MPNST was identified in 34 individuals (2%), yielding a relative risk value of 113. Lesions occurred in the limbs in 18 patients (53%), and those with limb lesions survived longer than those with nonlimb MPNSTs. Pain associated with a mass was the strongest suggestion of MPNST development.
Leroy et al. (2001) performed a retrospective study of MPNST in a cohort of 395 patients with NF1 followed for 11 years in a teaching hospital setting. Seventeen patients (4.3%) developed tumors, with a mean age at diagnosis of 32 years (SD = 14 years). Twelve patients had high-grade tumors; all tumors except 1 developed on preexisting nodular or plexiform neurofibromas. Pain and enlarging mass were the first and predominant signs. None of the benign tumors displayed significant p53 (TP53; 191170) staining or p53 mutations. Six of 12 malignant tumors significantly overexpressed p53, and 4 of 6 harbored p53 missense mutations. Median survival was 18 months overall, 53 months in peripheral locations, and 21 months in axial locations. Leroy et al. (2001) concluded that investigations and deep biopsy of painful and enlarging nodular or plexiform neurofibromas should be considered in patients with NF1, and that late appearance of p53 mutations and overexpression precludes their use as predictive markers for malignant transformation.
Evans et al. (2002) ascertained NF1 patients with MPNST in an attempt to assess lifetime risk. They found 21 NF1 patients who developed MPNST, equivalent to an annual incidence of 1.6 per 1,000 and a lifetime risk of 8 to 13%. There were 37 patients with sporadic MPNST. The median age at diagnosis of MPNST in NF1 patients was 26 years, compared to 62 years in patients with sporadic MPNST. In Kaplan-Meier analyses, the 5-year survival after diagnosis was 21% for NF1 patients with MPNST, compared to 42% for sporadic cases. One NF1 patient developed 2 separate MPNSTs in the radiation field of a previous optic glioma.
McCaughan et al. (2007) surveyed Scottish medical records across a 10-year period and identified 14 NF1 patients with a coexistent diagnosis of MPNST. The lifetime risk of developing MPNST was calculated to be 5.9 to 10.3%, and the mean age at diagnosis of the tumors was 42.1 years. Five-year survival after diagnosis of MPNST was significantly lower in NF1 patients compared to patients without NF1 (0% vs 54%, p less than 0.01).
Susceptibility to Other Malignancies
Crowe et al. (1956) found 6 secondary malignant lesions in 168 patients with neurofibromatosis. D'Agostino et al. (1963) discovered 21 cases of secondary neoplasms in his study of 678 cases of neurofibromatosis.
Knight et al. (1973) reviewed 69 patients with single and 45 patients with multiple neurofibromas. Five patients in the group were found to have a total of 11 secondary malignant lesions including 3 fibrosarcomas, 3 squamous cell carcinomas, and 1 neurofibrosarcoma, among other forms. Some earlier studies have reported mainly sarcomas associated with neurofibromatosis.
Clark and Hutter (1982) reported an apparent association between the rare entity juvenile chronic myelogenous leukemia and neurofibromatosis. They suggested that other types of nonlymphocytic leukemia have an increased frequency, but Riccardi (1982) raised the question as to whether these are families with only cafe-au-lait spots.
Kalff et al. (1982) found pheochromocytoma in 10 of 18 NF1 patients with hypertension. Age at diagnosis ranged from 15 to 62 years. The clinical characteristics of the neurofibromatosis did not predict the presence of pheochromocytoma. One patient without pheochromocytoma had coarctation of the aorta and 1 had renal artery stenosis; this patient was described as having the Turner phenotype. At least 2 of the pheochromocytoma patients had renal artery stenosis, and 3 had small bowel and/or stomach neurofibromata. One patient with pheochromocytoma also had hypernephroma with metastases and another had disseminated metastases from an undifferentiated leiomyosarcoma thought to originate from her upper gastrointestinal tract.
Voutsinas and Wynne-Davies (1983) suggested that the risk of malignancy in NF1 had been exaggerated and that the true value was 2.0% (or 4.2% of those over 21 years).
Crawford (1986) reported on a study of 116 NF1 patients under 12 years of age and reviewed the literature. Among the unusual presentations was rhabdomyosarcoma projecting from the urethra in a girl who also had congenital pseudarthrosis of the tibia. Crawford (1986) stated that 'most of the rhabdomyosarcomas associated with neurofibromatosis involve the genitourinary tract.'
Sayed et al. (1987) described malignant schwannoma in 3 brothers who had inherited neurofibromatosis from their mother. Two of the brothers had been reported by Herrmann (1950).
Griffiths et al. (1987) reported 9 cases of NF1 with a carcinoid tumor in the duodenum that had widespread somatostatin (SST; 182450) immunoreactivity. The duodenum was also the primary site in 18 of 20 published NF1 cases with carcinoid tumor. Pheochromocytoma was also present in 6 of the 27 cases with NF1 and duodenal carcinoid tumor. In cases of von Hippel-Lindau syndrome (193300), with which pheochromocytoma also occurs, Griffiths et al. (1987) found no carcinoid tumors, but did find islet cell tumor in association with pheochromocytoma. Swinburn et al. (1988) reported 2 patients with neurofibromatosis and duodenal carcinoid tumor, bringing the total number of cases of this association to 18. Their 2 cases as well as 5 others were positively identified as somatostatinomas. The histologic finding of psammoma bodies is important in the diagnosis of duodenal somatostatinomas. One patient also had a parathyroid adenoma, which was found postmortem.
Although NF1 has been called 'peripheral neurofibromatosis,' it has been associated with tumors of the central nervous system, which include astrocytomas of the visual pathways, ependymomas, meningiomas, and some primitive neuroectodermal tumors. The most common neuroimaging abnormality in NF1 is a high signal intensity lesion in the basal ganglia, thalamus, brainstem, cerebellum, or subcortical white matter referred to as an 'unidentified bright object' (UBO). These UBOs are thought to represent sites of vacuolar change. Molloy et al. (1995) studied 17 NF1 patients with brainstem tumors, which also presented increased T2 signal abnormality on MRI scanning. Fifteen of these 17 patients had neurologic signs and symptoms indicative of brainstem dysfunction and 35% of them had evidence of radiographic tumor progression. In the 2 patients that had partial surgical resection, pathology demonstrated either a fibrillary or anaplastic astrocytoma. As 15 of these 17 patients remained alive after a 52-month follow-up, this suggested that these are much less aggressive than typical pontine tumors which should be distinguished from the UBOs seen elsewhere in the brains of neurofibromatosis patients.
Hunerbein et al. (1996) described a 56-year-old man with NF1 who had had a 6-month history of recurrent epigastric pain and was found to have a multifocal malignant schwannoma of the duodenum causing biliary obstruction.
Sakaguchi et al. (1996) described a 48-year-old man with NF1 and paroxysmal hypertension in progressive respiratory insufficiency. Clinical investigation displayed calcified tumors in the anterior mediastinum and perirenal region. Histologic examination at autopsy revealed composite tumors consisting of pheochromocytoma and malignant peripheral nerve sheath tumor at 2 sites: the left adrenal gland and the region surrounding the inferior vena cava, probably corresponding to the right adrenal gland. In addition, the gastrointestinal tract was involved with mesenchymal tumors showing neurogenic differentiation.
Coffin et al. (2004) reviewed information indicating that children and young adults with NF1 have a higher risk for non-neurogenic sarcomas than the general population, in addition to an increased risk for malignant peripheral nerve sheath tumor. When non-neurogenic sarcomas occur in early childhood, a subsequent malignant peripheral nerve sheath tumor can occur as a second malignant neoplasm, especially after alkylating agent chemotherapy and irradiation. Coffin et al. (2004) presented 4 patients. In 1, embryonal rhabdomyosarcoma was diagnosed at the age of 2 years, and was treated by surgery, radiation, and chemotherapy. A malignant peripheral nerve sheath tumor was detected at the age of 13 years. A second patient likewise had the diagnosis of embryonal rhabdomyosarcoma at the age of 2 years and had the same therapy followed by T-cell lymphoblastic lymphoma at the age of 7 years.
Oguzkan et al. (2006) described 2 cases of NF1 with rhabdomyosarcoma. The first was that of an infant with overlapping phenotypic features of neurofibromatosis and Noonan syndrome (NS1; 163950) (see NFNS, 601321) who presented with rhabdomyosarcoma of the bladder. The second infant likewise exhibited NF1 features and was also associated with bladder rhabdomyosarcoma. Loss of heterozygosity (LOH) analysis of the NF1 gene using 7 intragenic markers and 1 extragenic polymorphic marker detected a deletion in the NF1 gene in the NFNS case associated with bladder rhabdomyosarcoma.
Bausch et al. (2006) reported that 15 (3%) of 565 pheochromocytoma cases in a pheochromocytoma registry had an NF1 mutation. In 10 additional cases contributed specifically for a study of pheochromocytoma in NF1, they found 92% had germline NF1 mutations. The 25 patients with NF1 were compared with patients with other syndromes associated with pheochromocytoma: 31 patients with multiple endocrine neoplasia type 2 (MEN2; 171400) due to mutation in the RET gene (164761); 21 patients with paragangliomas-1 (168000) due to mutation in the SDHD gene (602690); 33 patients with paragangliomas-4 (115310) due to mutation in the SDHB gene (185470); 75 patients with von Hippel-Lindau disease (193300) due to mutation in the VHL gene (608537); and 380 patients with pheochromocytoma as a sporadic disease. The characteristics of patients with pheochromocytoma related to NF1 were similar to those of patients with sporadic pheochromocytoma. There were significant differences between the NF1 group and the other respective groups in the age at diagnosis (von Hippel-Lindau disease and paragangliomas-1); in the extent of multifocal tumors (MEN2, von Hippel-Lindau disease, and paragangliomas-1); and in the extent of extraadrenal tumors (MEN2, von Hippel-Lindau disease, paragangliomas-1, and paragangliomas-4). Patients with NF1 had a relatively high (but not significant) prevalence of malignant disease (12%), second only to that among patients with paragangliomas-4 who had a germline mutation in the SDHB gene (24%). Taken together, 33% of all symptomatic patients with pheochromocytoma in the multicenter, multinational registry carried germline mutations in 1 of the 5 genes, including the NF1 gene.
Vascular Manifestations
Renal artery stenosis due to 'vascular neurofibromatosis' is a relatively common cause of hypertension in patients with NF1. Reubi (1945) first described vascular NF1. Involvement of the heart in neurofibromatosis was described and reviewed by Rosenquist et al. (1970), who also reviewed involvement of the abdominal aorta and renal, carotid, and other arteries.
Salyer and Salyer (1974) found peculiar arterial lesions in 7 of 18 autopsy cases of NF1 at the Johns Hopkins Hospital. They proposed that the pathogenesis of the arterial lesions was proliferation of Schwann cells within arteries with secondary degenerative changes, e.g., fibrosis, resulting in lesions with various appearances.
Among 40 pediatric patients (16 girls and 24 boys), aged 22 months to 17 years, undergoing operation for renovascular hypertension, Stanley and Fry (1981) found that 10 had neurofibromatosis, including 3 with abdominal aortic anomalies. Abdominal aortic coarctation affected 5 other children. Cure of the hypertension was achieved in 34 patients (85%); the condition was improved in 5; and one case was classified as a therapeutic failure. Single cases of renovascular hypertension in neurofibromatosis were reported by Allan and Davies (1970), Finley and Dabbs (1988), and others.
Brunner et al. (1974) described an unusual case of chronic mesenteric arterial insufficiency caused by vascular neurofibromatosis in a 50-year-old man with a 30-year history of chronic malabsorption and chronic small intestinal paralysis. He was said to have no signs of systemic disease or cafe-au-lait spots. Pigmentation of the perioral area and lips of the patient were attributed to longstanding malabsorption syndrome.
Zochodne (1984) reported a 16-year-old NF1 girl with aneurysm of the superior mesenteric artery complicating renovascular hypertension associated with coarctation of the abdominal aorta from above the celiac trunk to above the origin of the inferior mesenteric artery. The coarctation was associated with stenosis of the renal, celiac, and superior mesenteric arteries. The patient had typical skin signs of neurofibromatosis and had had a right below-knee amputation at age 5 for nonunion of a tibial fracture. The mother and 2 sibs were affected. A very similar patient with neurofibromatosis vasculopathy, or vascular neurofibromatosis, was reported by Lehrnbecher et al. (1994). The 4-year-old boy presented with congenital pseudarthrosis of the right tibia, suggesting the vascular origin of this well-known complication, multiple cafe-au-lait spots, short stature, and mild systemic arterial hypertension. The mother and grandmother had NF1. Subsequent complications of the vasculopathy were hypertension, septic infection of an aneurysm in the deltoid muscle, infarction of a segment of colon, sudden appearance of multiple arterial aneurysms, and venous thrombosis. Histologic examination of the bowel specimen confirmed the clinical diagnosis of vascular NF1: the proliferating cells seemed to have originated from myoblasts or myofibroblasts, and not from Schwann cells.
Craddock et al. (1988) reported a 24-year-old white woman with NF1 who had renovascular hypertension resulting from a proximal renal artery stenosis and poststenotic aneurysmal degeneration. Her sister, aged 38 years, presented similarly but without clinical evidence of neurofibromatosis.
Uren et al. (1988) found a congenital left atrial wall aneurysm in a patient with neurofibromatosis; however, the association may have been coincidental. Fitzpatrick and Emanuel (1988) observed the association of typical NF1 with hypertrophic cardiomyopathy in a brother and sister.
Kousseff and Gilbert-Barness (1989) reported what they referred to as 'vascular neurofibromatosis' in 2 patients who as infants developed idiopathic gangrene with vascular changes resembling those of NF1. An additional review of 105 patients uncovered a 27-month-old boy with NF1 and extensive vascular changes with renal hypertension. They discussed the possible relationship to arterial fibromuscular dysplasia. Stanley (1975) found that 5 of 25 children with arterial fibromuscular dysplasia had NF1 as well.
Nopajaroonsri and Lurie (1996) described venous aneurysm, arterial dysplasia, and near-fatal hemorrhages in a 62-year-old who was said to have familial neurofibromatosis (no family history was given). The patient presented with an aneurysm of the internal jugular vein which was associated with dysplasia of cervical arteries. Neurofibromatous tissue was found in the wall of the aneurysm as well as in small veins. During and after surgical excision of the aneurysm, the patient developed massive hemorrhages that required reexploration and evacuation of cervical hematomas. During surgery, bleeding was difficult to control because of excessive friability of blood vessels. Despite the vascular invasion by a tumor, there was no evidence of malignancy or malignant transformation in the patient after a 10-year follow-up.
Because neurofibromin is expressed in blood vessel endothelial and smooth muscle cells, Hamilton and Friedman (2000) suggested that NF1 vasculopathy may result from an alteration of neurofibromin function in these cells.
Riccardi (2000) supported the view that endothelial injury and its repair, which appear to be important in the pathogenesis of atherosclerosis, may also play a role in NF1 vasculopathy. He recommended a regimen of aggressive antihypertensive treatment of children with NF1 in whom either episodic or persistent systemic hypertension is documented. The goal would be to decrease intravascular trauma, based on the supposition that such trauma is directly related to the evolution of the vascular disease in patients with NF1.
Lin et al. (2000) reviewed cases of NF1 and cardiovascular malformations among 2,322 patient records in the National Neurofibromatosis Foundation International Database, collected between 1991 and 1998. Cardiovascular malformations were reported in 54 (2.3%) of the NF1 patients, 4 of whom had Watson syndrome (193520) or neurofibromatosis-Noonan syndrome (NFNS; 601321). Of the 54 patients, 25 had pulmonic stenosis, and 5 had coarctation of the aorta, representing a higher proportion of all cardiovascular malformations than expected. The authors recommended that all individuals with NF1 have careful cardiac auscultation and blood pressure monitoring as part of every NF-related examination.
Hamilton et al. (2001) reported a previously healthy 33-year-old man with NF1 who died suddenly. Autopsy revealed multiple cardiac abnormalities, including evidence of an intramyocardial vasculopathy characteristic of the vascular pathology found in NF1. Other cardiac findings included nonspecific cardiomyopathic changes, myocardial fibrosis, and a floppy mitral valve. The authors emphasized the importance of recognition of vascular lesions in patients with NF1 so that appropriate management can be provided.
Friedman et al. (2002) reviewed cardiovascular disease in NF1. The NF1 Cardiovascular Task Force suggested that all patients with NF1, especially those with Watson or NF1-Noonan phenotypes, have a careful cardiac examination with auscultation and blood pressure measurement.
Tomsick et al. (1976) reported intracranial arterial occlusive disease in NF1. Erickson et al. (1980) described 2 sisters with neurofibromatosis and intracranial arterial occlusive disease leading to the moyamoya pattern of collateral circulation (MYMY1; 252350). Four other members of their sibship of 8, and members of 2 previous generations, including the mother, had neurofibromatosis. Yamauchi et al. (2000) stated that more than 50 cases of the association of NF1 and moyamoya disease had been described, including the cases reported by Woody et al. (1992) and Barrall and Summers (1996). See MYMY2 (607151) for a form of moyamoya disease showing linkage to chromosome 17q25.
Benatar (1994) described a 27-year-old man with neurofibromatosis who presented with 3 intracranial fusiform aneurysms. He referred to 3 previous descriptions of large intracranial fusiform aneurysms in patients with NF1, which he considered to be considerably less common than renal and gastrointestinal vascular lesions in this disorder.
Schievink et al. (2005) detected incidental intracranial aneurysms in 2 (5%) of 39 patients with NF1 who were hospitalized for other reasons. Limiting the patient population to the 22 patients who had a brain MRI resulted in a significantly higher detection rate of 9% compared to 0% in 526 control patients with primary or metastatic brain tumors who underwent brain MRI. The findings suggested that patients with NF1 are at an increased risk of developing intracranial aneurysms as a vascular manifestation of NF1.
Central Nervous System Abnormalities
Adornato and Berg (1977) observed the diencephalic syndrome in 2 infants who had neurofibromatosis and hypothalamic tumors.
Horwich et al. (1983) presented evidence that aqueductal stenosis occurs in neurofibromatosis.
Senveli et al. (1989) reported 6 patients with NF1 who had aqueductal stenosis and hydrocephalus requiring surgical intervention. Ages varied from 14 to 24 years. Twenty-two similar cases were found in the literature.
Winter (1991) described dural ectasia in neurofibromatosis causing bony erosion that was sufficiently severe to destroy spinal stability. Eichhorn et al. (1995) described dural ectasia in a 20-year-old woman with NF1 who presented with back and leg pain. Increasingly severe back pain led to investigations which showed multiple fractures of the pedicles of L1 to L4 with dural ectasia penetrating the body of L2. The transverse diameter of the dura was twice that of the vertebral body at that level, reaching and lifting the psoas.
Mukonoweshuro et al. (1999) reviewed the central nervous system manifestations and neuroradiologic findings in NF1.
Skeletal Manifestations
Skeletal abnormalities in NF1 include short stature, scoliosis, sphenoid wing dysplasia, and tibial pseudarthrosis, a bowing of the long bone that looks like a false joint (reviews by Ferner et al., 2007 and Williams et al., 2009).
Konishi et al. (1991) described a 40-year-old woman with NF1 and typical hypophosphatemic osteomalacia. Bone pain, multiple pseudofractures, marked increase in osteoid by bone biopsy, and hypophosphatemia with renal phosphate wasting were features. Treatment with oral phosphate and vitamin D was effective. They found reports of 34 similar cases and pointed out that of the 67 patients collected by Dent (1952), 2 had neurofibromatosis.
In a father and 3 children by 2 different women, Schotland et al. (1992) described cosegregation of NF1 and osseous fibrous dysplasia. In the 4 individuals with NF1, cafe-au-lait spots and neurofibromata were present in all 4, Lisch nodules and macrocrania in 3, and scoliosis and curvature of the long bones in 2. Schotland et al. (1992) found at least 8 reports of NF1 and osseous fibrous dysplasia associated in individuals but no previous description of a familial association. The osseous dysplasia consisted of multiple lesions at the distal ends of the shafts of the femurs and in the tibias and fibulas, with bowing of the fibulas.
Stevenson et al. (1999) reported a descriptive analysis of tibial pseudarthrosis in a large series of NF1 patients. A male predominance was observed among patients with pseudarthrosis, leading the authors to suggest that male gender may be a susceptibility factor. Examination of the natural history of pseudarthrosis showed that half of the patients who had a fracture sustained it before age 2 years, and that approximately 16% of the pseudarthrosis patients had an amputation.
Long bone dysplasia, seen in 2% (Ferner et al., 2007) to 5% (Stevenson et al., 2006) of patients with NF1, typically involves the tibia and frequently presents with anterolateral bowing that may progress to fracture and nonunion. Tibial dysplasia is most often unilateral, evident in the first year of life, and usually not associated with a neurofibroma at the site, suggesting a random molecular event. Stevenson et al. (2006) documented double inactivation of the NF1 gene in pseudarthrosis tissue, and suggested involvement of the neurofibromin-Ras signal transduction pathway. Prospectively acquired tissue from the pseudarthrosis site of 2 individuals with NF1 did not show typical immunohistochemical features of neurofibroma, but genetic markers spanning the NF1 locus demonstrated loss of heterozygosity. Patient 1 of Stevenson et al. (2006) was a 42-year-old man with a father with NF1 and a brother with NF1 associated with lower limb pseudarthrosis requiring amputation. Patient 2 was a 2-year-old boy whose tibial and fibular bowing presented at birth, with subsequent fibular fracture at age 2 weeks. Clinical findings consistent with NF1 included more than 5 cafe-au-lait macules and tibial pseudarthrosis. The mother had NF1.
Lammert et al. (2006) found significantly lower mean serum levels of 25-hydroxyvitamin D in 55 NF1 patients compared to controls (14.0 ng/ml in patients, 31.4 ng/ml in controls). Among the NF1 patients, there was a highly significant inverse correlation between serum vitamin D concentration and the number of dermal neurofibromas. Lammert et al. (2006) noted that focal osseous abnormalities and decreased bone mineral density are observed in patients with NF1, which may be related to inadequate circulating vitamin D. The relationship of serum vitamin D to neurofibromas was unclear.
Cognitive and Neuropsychologic Manifestations
Twelve NF1 families with 1 affected child, an unaffected sib, and both natural parents were studied by Hofman et al. (1994) to assess the presence of cognitive deficits or learning disability. NF1 children with known intracranial problems were excluded, but family members with known learning disabilities or hyperactivity disorders were not, making some of the results difficult to interpret. Full scale IQs ranged from 70 to 130 among children with NF1 and from 99 to 139 among unaffected sibs. Scores of parents with NF1 ranged from 85 to 114 compared to 80 to 134 in unaffected parents. Children with NF1 showed significant deficits in language and reading abilities compared to sibs, but not in mathematics. They also had impaired visuospatial and neuromotor skills. In 11 of 12 NF1 children but in none of the unaffected sibs, foci of high signal intensity on T2-weighted MRI scan images were observed. A statistically significant correlation was found between lowering of IQ and visuospatial deficits and the number of foci seen on scan.
Legius et al. (1994) studied the neuropsychologic profiles of 46 children with NF1. They found a reduction in total IQ, but a significantly better verbal rating than performance rating in all age groups. Concentration problems were especially significant in children with a higher IQ. Legius et al. (1994) suggested that these children may benefit from the use of Ritalin.
T2 'unidentified bright objects' are seen in 50 to 75% of children with NF1, most frequently in the basal ganglia, corpus cerebellum, and brainstem. Legius et al. (1995) found no difference in the mean intelligence of 18 children with such lesions and 10 neurofibromatosis children who did not show such lesions.
Silva et al. (1997) stated that learning disabilities are said to occur in 30 to 45% of patients with NF1, even in the absence of any apparent neuropathology. The learning disabilities may include a depression in mean IQ scores, visuoperceptual problems, and impairment in spatial cognitive abilities.
Schrimsher et al. (2003) found an association