Hereditary Hemorrhagic Telangiectasia
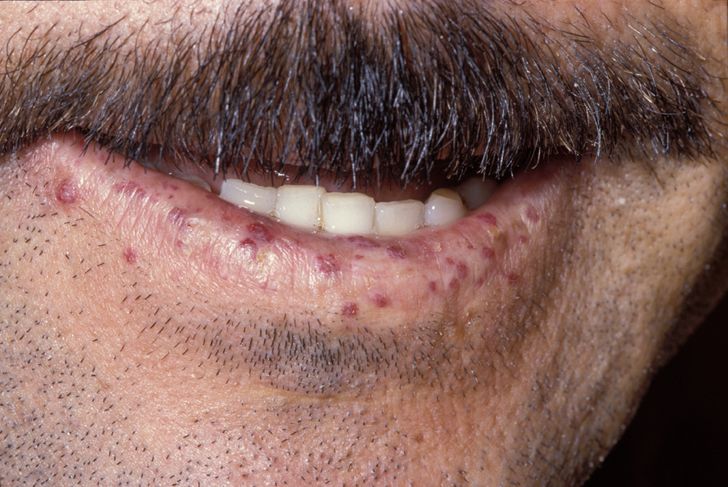
An inherited disorder of angiogenesis characterized by mucocutaneous telangiectases and visceral arteriovenous malformations.
Epidemiology
The prevalence is approximately 1/6,000
Clinical description
The most common clinical signs of hereditary hemorrhagic telangiectasia (HHT) include recurrent epistaxis (nosebleeds), frequently from childhood, and cutaneous or mucosal telangiectases generally presenting later, and increasing with age, where anemia may become an important part of the disease. Visceral arteriovenous malformations (AVMs) are usually asymptomatic but can lead to complications that produce highly variable manifestations. The age of onset of AVM-related complications is variable, ranging from childhood to geriatric age, with a few cases reported during the neonatal period. Pulmonary AVMs may manifest with brain abscesses, strokes, transient ischemic attacks, signs of chronic hypoxaemia or, rarely, haemorrhagic rupture. AVMs of the central nervous system can be haemorrhagic or, rarely, produce signs of slow compression. Hepatic AVMs, which can remain latent for a long time, in a limited proportion of patients become severe leading to high-output cardiac failure, portal hypertension, pulmonary hypertension or ischemic cholangitis. Hemorrhagic digestive telangiectases increase with age and can worsen chronic anemia.
Etiology
This genetic disorder is due to pathogenic variants primarily in ENG (9q34.11) or ACVRL1 (12q13.13), encoding proteins involved in vascular development and angiogenic homeostasis of capillaries. Mutations in SMAD4 (18q21.2) occur in rare cases (1-3%) and result in HHT associated with juvenile polyposis. In a small proportion of HHT families, the pathogenic gene variant has not yet been identified.
Diagnostic methods
The diagnosis is clinical and/or molecular. The clinical diagnosis is based on having at least three of the four Curaçao criteria: recurrent epistaxis, cutaneous/mucosal telangiectases, visceral involvement, and a first line family member with HHT. Genetic testing can be used to screen, to confirm a diagnosis, or to rule out the diagnosis if the pathogenic variant is known in the family.
Differential diagnosis
The differential diagnosis includes limited cutaneous systemic sclerosis, digestive angiodysplasias, isolated sporadic AVMs in the lungs, liver and brain, other vascular anomaly syndromes that cause AVMs; benign hereditary telangiectasia; and other causes of recurrent epistaxis (coagulation disorders or other local nasal factors).
Antenatal diagnosis
Prenatal genetic testing is possible in families where the pathogenic variant has been identified in the family, but is not necessary for proper pregnancy and delivery management. Decisions about prenatal genetic testing are the choice of the parents, but discussion of all related issues is appropriate. The usual antenatal scans will be offered, and sonographers aware of the presence of HHT in the family will detect most major AVMs.
Genetic counseling
Transmission is autosomal dominant. Penetrance is age dependent, the majority having symptoms before 50 years of age. The phenotype is highly variable, even between members of the same family.
Management and treatment
Disease management includes prevention and treatment of epistaxis and anaemia, screening for AVMs, and guidance regarding pregnancy-related issues. The management of pulmonary AVM(s) relies on early detection, occlusion where feasible, and ongoing care in cases of persistent pulmonary AVMs. For severe liver involvement, multidisciplinary patient assessment in a center with expertise in HHT is recommended. Usually, cerebral AVMs that have not bled are not treated, whereas cerebral AVMs that have already bled or have become symptomatic usually require treatment. Gastrointestinal telangiectases may sometimes be the cause of significant anemia, especially in older patients, and need specific management. Awareness of the possibility of AVMs/HHT is important for optimal management of diverse medical states. HHT due to a SMAD4 pathogenic variant requires polyposis screening and aortic follow up.
Prognosis
Life expectancy is reduced in unscreened patients. In patients assessed and treated for pulmonary AVMs in an HHT Center, life expectancy is comparable to the general population. Pregnancy-related death has been reported, and is a particular risk for women with pulmonary arteriovenous malformations.