Trimethylaminuria
A number sign (#) is used with this entry because of evidence that trimethylaminuria, sometimes referred to as fish-odor syndrome, is caused by homozygous or compound heterozygous mutation in the gene encoding flavin-containing monooxygenase-3 (FMO3; 136132) on chromosome 1q24.
Another inborn error of metabolism accompanied by fish-like body odor results from deficiency of dimethylglycine dehydrogenase (see 605850).
DescriptionTrimethylaminuria results from the abnormal presence of large amounts of volatile and malodorous trimethylamine within the body. This chemical, a tertiary aliphatic amine, is excreted in the urine, sweat (ichthyohidrosis), and breath, which take on the offensive odor of decaying fish (Mitchell, 1996).
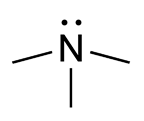
Clinical FeaturesIndividuals with trimethylaminuria excrete relatively large amounts of amino-trimethylamine (TMA) in their urine, sweat, and breath, and exhibit a fishy body odor characteristic of the malodorous free amine, leading to the designation fish-odor syndrome. TMA is a product of intestinal bacterial action. The substrates from which it is derived are choline, which, bound to lecithin, is present most abundantly in egg yolk, liver, kidney, legumes, soy beans, and peas, as well as from trimethylamine-N-oxide, a normal constituent of saltwater fishes. Normally, TMA produced in the gut is absorbed and oxidized in the liver by FMO, a microsomal mixed-function oxidase (Higgins et al., 1972).
Humbert et al. (1970) first used the terms trimethylaminuria and fish-odor syndrome to describe a 6-year-old girl who intermittently had a fishy odor. She also had multiple pulmonary infections beginning in the neonatal period, the clinical stigmata of Turner syndrome but normal karyotype, splenomegaly, anemia, and neutropenia. Her urine contained increased amounts of TMA. In the same patient, Humbert et al. (1971) found defective membrane function in platelets, neutrophils, and red cells, and Higgins et al. (1972) found deficiency of trimethylamine oxidase by liver biopsy. Calvert (1973) noted that the features in the patient of Humbert et al. (1970) were those of Noonan syndrome (163950). He studied a clinically identical patient but found no trimethylaminuria with or without loading with trimethylamine. Witt et al. (1988) included the patient of Humbert et al. (1970) in their series of cases of Noonan syndrome with bleeding diathesis.
Lee et al. (1976) observed a brother and sister with trimethylaminuria; in both, an offensive fishy odor occurred when the mother was breast feeding them and had eaten eggs or fish. Danks et al. (1976) referred to 4 affected individuals in their personal experience.
Mayatepek and Kohlmuller (1998) described 2 unrelated children with transient trimethylaminuria. One was a 2-month-old female infant referred because of an offensive odor on her skin and from her urine which was noticed by the parents. When the child was 6 months old, the fishy odor completely disappeared. The second patient was a 4-year-old boy who was referred because of smelly urine and skin which had been noticed by his mother from about the age of 18 months. In these children, transient trimethylaminuria occurred without N-oxidation deficiency.
Zschocke et al. (1999) studied patients with mild trimethylaminuria and concluded that FMO3 deficiency is a spectrum of phenotypes that can include transient or mild malodor depending on environmental exposures. Mild FMO3 deficiency may have clinical relevance beyond intermittent body odor leading to an abnormal metabolism of drugs, hypertension, or increased cardiovascular disease risk.
Todd (1979) noted that patients with TMA may be deeply disturbed, depressed, and even suicidal, with psychosocial problems in school. Rehman (1999) also reported that patients with TMA often have psychosocial problems, including strong feelings of shame, embarrassment, low self-esteem, social isolation, anxiety, and depression.
Clinical ManagementTreatment for trimethylaminuria can involve counseling, dietary adjustments, short-course treatment with metronidazole, neomycin, or lactulose, and the use of soaps with a pH value of 5.5-6.5 (Rehman, 1999).
InheritanceAyesh et al. (1993) studied 187 subjects with suspected body malodor and concluded that the trimethylaminuria is inherited as an autosomal recessive trait.
DiagnosisAl-Waiz et al. (1987, 1988) presented evidence for deficiency in the N-oxidation of trimethylamine in persons with trimethylaminuria. The parents of affected persons showed partial impairment of N-oxidation on substrate challenge. They found 2 possible carriers among 169 randomly screened persons. N-oxidation is an important route of biotransformation for many substances including nicotinamide, nicotine, guanethidine, and metyrapone. Al-Waiz et al. (1989) described a TMA loading test for detection of carriers. Zhang et al. (1995) confirmed the oral trimethylamine challenge test for the identification of heterozygotes. Among 100 apparently normal volunteers who were challenged with trimethylamine, 1 had an N-oxidation capacity that fell within the range found among obligate heterozygotes.
Ayesh et al. (1993) studied 187 subjects with suspected body malodor ascertained in response to a newspaper story concerning the fish-odor syndrome. Biochemical tests were performed in 156 of the patients and 5 families of 6 of the subjects with the fish-odor syndrome agreed to further tests. The fish-odor syndrome was diagnosed in 11 subjects; the percentage of total trimethylamine excreted in their urine samples that was oxidized to trimethylamine N-oxide was less than 55% under normal dietary conditions and less than 25% after oral challenge with trimethylamine. In normal subjects, more than 80% of trimethylamine was N-oxidized. All parents of 6 subjects with the syndrome who were tested showed impaired N-oxidation of excreted trimethylamine after oral challenge, indicating that they were heterozygous carriers of the allele for the syndrome.
Mayatepek and Kohlmuller (1998) found that transient trimethylaminuria in 2 children occurred without N-oxidation deficiency. This demonstrated that a diagnosis of fish-odor syndrome should include the analysis of urinary excretion not only of trimethylamine but also of trimethylamine-N-oxide.
Molecular GeneticsAkerman et al. (1997) and Dolphin et al. (1997) demonstrated that trimethylaminuria is caused by mutation in the FMO3 gene (136132). One individual of British extraction was shown to be homozygous for an E305X mutation (136132.0001) of the FMO3 gene; this person, in addition to trimethylaminuria, had tachycardia and severe hypertension after eating cheese (which contains tyramine) and after using nasal epinephrine following an epistaxis (Danks et al., 1976). The FMO3 enzyme metabolizes tyramine.
Zschocke et al. (1999) examined the patients of Mayatepek and Kohlmuller (1998) with transient trimethylaminuria and other patients with mild trimethylaminuria and found compound heterozygosity for a missense mutation on one allele and 2 amino acid polymorphisms (E158K, E308G) on the other allele (see, e.g., 136132.0015). Zschocke et al. (1999) found that the variant allele with the 2 polymorphisms occurred in 20% and 6% of German and Turkish controls, respectively. The authors performed standardized TMA challenge tests in the controls with this variant allele and found markedly reduced FMO3 enzyme activity in vivo.
HistoryReports of fish-like odor in people have been found in literature as far back as 1400-1000 B.C. in the Indian epic of the Bharata Dynasty, 'Mahabharata,' by Vyasa (Mitchell, 1996).