Leber Congenital Amaurosis 13
A number sign (#) is used with this entry because Leber congenital amaurosis-13 (LCA13) is caused by homozygous or compound heterozygous mutation in the photoreceptor-specific retinal dehydrogenase gene RDH12 (608830) on chromosome 14q24.
Heterozygous or homozygous mutation in RDH12 has also been shown to cause a form of retinitis pigmentosa (RP53).
For a general phenotypic description and a discussion of genetic heterogeneity of Leber congenital amaurosis, see 204000; for retinitis pigmentosa, see 268000.
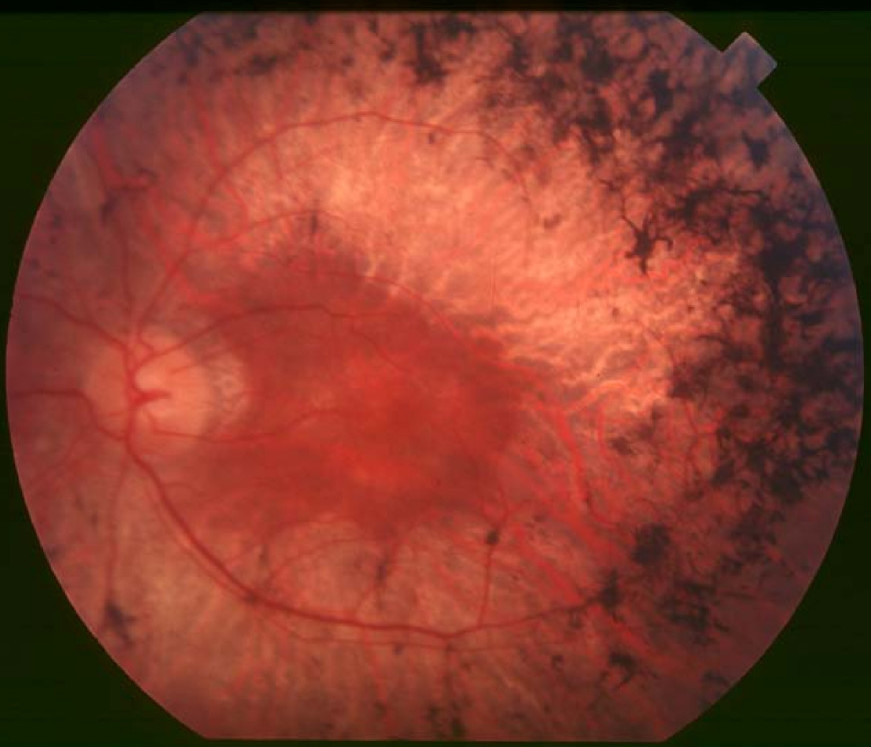
Clinical FeaturesJanecke et al. (2004) reported 3 consanguineous Austrian kindreds segregating Leber congenital amaurosis. Affected individuals in these families, as well as 2 Austrian individuals with sporadic LCA and 3 non-Austrian individuals with LCA, carried mutations in the RDH12 gene (see MOLECULAR GENETICS). All patients harboring RDH12 mutations had a severe yet progressive rod-cone dystrophy with severe macular atrophy but no or mild hyperopia. All individuals with RDH12-related LCA demonstrated onset of symptoms in early childhood (2-4 years) and progression to legal blindness in early adulthood (18-25 years). Both rods and cones were affected. Fundi showed pronounced attenuation of retinal arterioles and intraretinal bone spicule pigmentation. The electroretinogram was extinguished at the time of first investigation (as early as 5 and 6 years of age in 2 of the individuals).
In a study of 4 patients with LCA13, Jacobson et al. (2007) found that the retinal architecture was appreciably distorted, precluding identification of the normal laminae. Although some of the RDH12-mutant retinas were remarkably thick and others thin, all had the same dysplastic pattern detectable in the first decade of life. The authors noted that these findings contrast with those in RPE65 (180069)-mutant retinas in LCA2 (204100) in which the retinal structure is relatively preserved.
Retinitis Pigmentosa 53
Fingert et al. (2008) studied 35 members of a 6-generation family segregating autosomal dominant retinitis pigmentosa and identified 19 clinically affected individuals who had retinal findings typical of RP, including intraretinal bone spicule-like pigmentation and attenuation of retinal arterioles. The average age at diagnosis was 28.5 years (range, 12-43 years). Some family members maintained excellent central visual acuity and driving privileges into their eighth decade of life.
Benayoun et al. (2009) described a large, highly consanguineous pedigree segregating autosomal recessive early-onset retinitis pigmentosa, affecting 17 individuals from 10 sibships. The age at diagnosis ranged from 18 months to 35 years, but most were diagnosed within the first decade of life. Most patients reported poor night vision, reduced central vision, and peripheral field loss. Both scotopic and photopic ERGs were markedly reduced or completely extinct, but there was some degree of phenotypic variability. Funduscopic findings in most individuals included typical bone spicule-type pigment deposits, attenuation of the retinal arterioles, and pale appearance of the optic disc.
MappingJanecke et al. (2004) performed a whole-genome scan in 3 consanguineous Austrian families segregating LCA and identified a founder haplotype and a critical interval of 1.53 cM on chromosome 14q23.3-q24.1 that overlapped with the LCA3 locus (604232).
Retinitis Pigmentosa 53
In a large 6-generation family segregating autosomal dominant retinitis pigmentosa, Fingert et al. (2008) performed genomewide linkage analysis in 8 affected individuals and identified a 15.2-Mb region of chromosome 14q with a maximum nonparametric multipoint linkage score of 19.97. Genotyping all 19 affected individuals from the pedigree confirmed the linkage and yielded a maximum 2-point lod score of 6.81 (theta = 0.0) with marker D14S587. Analysis of recombination events narrowed the critical region to a 21.7-cM (18.6-Mb) interval between markers D14S1018 and D14S251 containing 173 genes.
In a large, highly consanguineous pedigree segregating autosomal recessive early-onset retinitis pigmentosa, Benayoun et al. (2009) performed haplotype analysis in 1 sibship using markers linked to all known autosomal recessive RP loci and genes and found linkage to chromosome 14q24.1 (haplotype 'A;' maximum 2-point lod score of 3.0 for marker D14S1069 at theta = 0.0). Haplotype A segregated with disease in 8 of 10 sibships, but 3 affected individuals in 2 sibships did not carry haplotype A.
Molecular GeneticsIn all affected family members studied from 3 kindreds with LCA13, as well as in 2 Austrian individuals with sporadic LCA13, Janecke et al. (2004) identified homozygosity for a tyr226-to-cys mutation in the RDH12 gene (Y226C; 608830.0001). Janecke et al. (2004) identified additional mutations in RDH12 in 3 of 89 non-Austrian individuals with LCA13: 806delCCCTG (608830.0002) and gln189 to ter (Q189X: 608830.0003), each in homozygous state, and thr49 to met (T49M; 608830.0004) and arg62 to ter (R62X; 608830.0005) in compound heterozygosity.
Perrault et al. (2004) studied a series of 110 unrelated patients with LCA; in 4.1% of the patients, they identified mutations in the RDH12 gene (see 608830.0001-608830.0002, 608830.0006-608830.0013). All patients harboring RDH12 mutations had a severe yet progressive rod-cone dystrophy with severe macular atrophy but no or mild hyperopia; Perrault et al. (2004) noted that this phenotype may represent the upper extreme of the spectrum of retinitis pigmentosa (RP; see 268000).
In a cohort of 1,011 individuals diagnosed with autosomal recessive retinal dystrophy, Thompson et al. (2005) identified 20 different disease-associated RDH12 mutations in a total of 22 individuals. Haplotype analysis suggested a founder mutation for each of the 3 common mutations: 806_810delCCCTG (608830.0002), L99I (608830.0010), and T155I (608830.0014). Patients typically presented with early disease that affected the function of both rods and cones and progressed to legal blindness in early adulthood.
Retinitis Pigmentosa 53
In a large 6-generation family segregating autosomal dominant retinitis pigmentosa mapping to chromosome 14q, Fingert et al. (2008) sequenced the candidate gene RDH12 and identified heterozygosity for a 1-bp deletion (608830.0015) in 19 affected individuals that was not found in unaffected family members or in 158 controls.
In a large, highly consanguineous pedigree segregating autosomal recessive early-onset retinitis pigmentosa, Benayoun et al. (2009) identified homozygosity for a missense mutation (A126V; 608830.0016) in 14 affected individuals from 8 sibships. The mutation was not found in 3 affected individuals from 2 additional sibships; in 2 of those patients, retinal pigmentation was milder and had an atypical appearance. Because haplotype analysis had excluded RDH12 as the gene underlying RP in these 2 sibships, Benayoun et al. (2009) concluded that the phenotype in these individuals was caused by mutation in another gene. A 45-year-old male family member who was heterozygous for A126V appeared to have a subclinical phenotype: although he did not describe significant subjective visual difficulties and denied nyctalopia or photosensitivity, his photopic ERG was at the lower limit of the normal range and his scotopic ERG was markedly reduced. Benayoun et al. (2009) noted that mild clinical phenotypes, including reduced ERG responses, had previously been reported in carriers of mutations in various RP genes.
NomenclatureThe form of LCA caused by mutation in the RDH12 gene, LCA13, was originally designated LCA3 because the RDH12 gene mapped within the region identified in a family with LCA3 reported by Stockton et al. (1998). It was later shown that the LCA3 form (604232) is not caused by mutation in the RDH12 gene and represents a separate LCA locus.