Familial Hypercholesterolemia
Summary
Clinical characteristics.
Familial hypercholesterolemia (FH) is characterized by severely elevated LDL cholesterol (LDL-C) levels that lead to atherosclerotic plaque deposition in the coronary arteries and proximal aorta at an early age, leading to an increased risk for cardiovascular disease. Xanthomas (patches of yellowish cholesterol buildup) may worsen with age as a result of extremely high cholesterol levels. Xanthomas can occur around the eyelids and within the tendons of the elbows, hands, knees, and feet. In FH, the more common cardiovascular disease is coronary artery disease (CAD), which may manifest as angina and myocardial infarction; stroke occurs more rarely. Untreated men are at a 50% risk for a fatal or nonfatal coronary event by age 50 years; untreated women are at a 30% risk by age 60 years.
An estimated 70%-95% of FH results from a heterozygous pathogenic variant in one of three genes (APOB, LDLR, PCSK9). FH is the most common inherited cardiovascular disease, with a prevalence of 1:200-250. FH likely accounts for 2%-3% of myocardial infarctions in individuals younger than age 60 years.
In contrast, homozygous FH (HoFH) results from biallelic (homozygous or compound heterozygous) pathogenic variants in one of these known genes (APOB, LDLR, PCSK9). Most individuals with HoFH experience severe CAD by their mid-20s and the rate of either death or coronary bypass surgery by the teenage years is high. Severe aortic stenosis is also common.
Diagnosis/testing.
Several formal diagnostic criteria exist for FH. The diagnostic criteria most widely used in Western countries include: extreme hypercholesterolemia (untreated adults with LDL-C>190 mg/dL or total cholesterol levels >310 mg/dL; untreated children/adolescents with LDL-C levels >160 mg/dL or total cholesterol levels >230 mg/dL); history of premature CAD or other CVD; xanthomas; corneal arcus; and a family history of features suggestive of FH. The diagnosis of FH can also be established by identification of a heterozygous pathogenic variant in one of the three genes (APOB, LDLR, and PCSK9) known to be associated with FH.
The diagnosis of HoFH can be established in a proband by identification of biallelic pathogenic variants in one of the three genes (APOB, LDLR, and PCSK9) known to be associated with FH.
Management.
Treatment of manifestations: Adults with FH: reduce CAD risk factors including cessation of smoking, regular physical activity, healthy diet, and weight control; treatment of hypertension; low-dose aspirin in high-risk individuals; pharmacotherapy (statins with additional medications as needed) to reduce lipid levels; referral to a lipid specialist if necessary to reduce LDL-C levels. Children with FH: referral to a lipid specialist; diet and lifestyle modifications; statins can be used in children starting around age eight years. Children and adults with HoFH: referral to a lipid specialist or specialized center for management of multiple drug therapy; LDL apheresis is often required; liver transplantation in rare circumstances.
Prevention of primary manifestations: Statin-based therapy with addition of other medications as needed, in combination with a heart-healthy diet (including reduced intake of saturated fat and increased intake of soluble fiber to 10-20 g/day); increased physical activity; not smoking.
Surveillance: Children with an established diagnosis of FH or risk factors for FH (e.g., elevated serum cholesterol, a family history of FH, a family history of premature CAD or other CVD) should have lipid levels checked before age ten years. All individuals with FH should have lipid levels monitored as recommended. Individuals with HoFH should be monitored with various imaging modalities (including echocardiograms, CT angiograms, and cardiac catheterization) as recommended.
Agents/circumstances to avoid: Smoking, high intake of saturated and trans unsaturated fat, excessive intake of cholesterol, sedentary lifestyle, obesity, hypertension, and diabetes mellitus.
Evaluation of relatives at risk: Early diagnosis and treatment of first-degree and second-degree relatives at risk for FH can reduce morbidity and mortality. The genetic status of at-risk family members can be clarified by either: (1) molecular genetic testing if the pathogenic variant has been identified in an affected family member; or (2) measurement of LDL-C concentration.
Pregnancy management: Pregnant women should incorporate all the recommended lifestyle changes including low saturated fat intake, no smoking, and high dietary soluble fiber. Statins are contraindicated in pregnancy due to concerns for teratogenicity and should be discontinued prior to conception. Bile acid binding resins (e.g., colesevelam) are generally considered safe (Class B for pregnancy), and LDL apheresis is also occasionally used if there is evidence of established CAD.
Genetic counseling.
Heterozygous familial hypercholesterolemia (FH) and homozygous familial hypercholesterolemia (HoFH) are inherited in an autosomal dominant manner.
Almost all individuals diagnosed with FH have an affected parent; the proportion of FH caused by a de novo pathogenic variant is unknown but appears to be extremely low. Each child of an individual with FH has a 50% chance of inheriting the pathogenic variant.
If both parents have FH, each child has a 50% chance of having FH, a 25% chance of having HoFH, and a 25% chance of not having FH.
If the pathogenic variant has been identified in a family member with FH (or if both pathogenic variants have been identified in a family member with HoFH), prenatal testing for pregnancies at increased risk is possible.
Diagnosis
In this GeneReview:
- Familial hypercholesterolemia (FH) refers to hypercholesterolemia resulting from a heterozygous pathogenic variant in one of several genes (APOB, LDLR, and PCSK9); it is also referred to as heterozygous FH (HeFH). FH is a relatively common disorder (prevalence 1:200-1:250).
- Homozygous FH (HoFH) refers to familial hypercholesterolemia resulting from biallelic (including true homozygous and compound heterozygous) pathogenic variants in one of these same genes (APOB, LDLR, and PCSK9). HoFH is much rarer than FH (prevalence 1:160,000 to 1:250,000 [Nordestgaard et al 2013, Cuchel et al 2014].
Suggestive Findings
Familial hypercholesterolemia (FH) should be suspected in individuals with the following findings:
- Extreme hypercholesterolemia
- Adults (untreated):
- Low-density lipoprotein cholesterol (LDL-C) levels >190 mg/dL (>4.9 mmol/L)
- Total cholesterol levels >310 mg/dL (>8 mmol/L)
- Children/adolescents (untreated):
- LDL-C levels >130 mg/dL (>3.4 mmol/L)
- Total cholesterol levels >230 mg/dL (>6 mmol/L)
- History of premature coronary artery disease (CAD) or other cardiovascular disease (CVD) (e.g., angina pectoris, myocardial infarction, peripheral vascular disease)
- Physical examination findings (e.g., xanthomas, corneal arcus)
- Family history of premature CAD and/or CVD
Establishing the Diagnosis
Currently three formal diagnostic criteria for FH are widely used in Western countries: the MEDPED Criteria, the Simon Broom Criteria, and the FH Dutch Lipid Clinic Criteria. (See Harada-Shiba et al [2012] for criteria used in non-Western countries.)
All three criteria rely on a combination of the following:
- Extreme hypercholesterolemia
- History of premature CAD or other CVD
- Findings on physical examination
- Family history of premature CAD or other CVD
- Identification of a pathogenic variant in a gene known to be associated with FH (see Table 1)
Extreme hypercholesterolemia
- Adults (untreated):
- LDL-C levels >190 mg/dL (>4.9 mmol/L)
- Total cholesterol levels >310 mg/dL (>8 mmol/L)
- Children or adolescents (untreated):
- LDL-C levels >160 mg/dL (>4 mmol/L)
- Total cholesterol levels >230 mg/dL (>6 mmol/L)
Note: (1) A non-fasting lipid panel can be obtained first in the initial evaluation of children, and, if abnormal or borderline, a fasting LDL-C level should be obtained [Martin et al 2013]. Elevation of two consecutive LDL-C levels is often recommended to confirm the diagnosis. (2) Age-specific LDL-C or total cholesterol levels are more specific in determining the likelihood of FH; for instance, LDL-C or total cholesterol levels >95th percentile for age, gender, and country [Starr et al 2008, Nordestgaard et al 2013]. (3) Computer (including mobile or smart phone-based) applications (see FH Diagnosis) can assist with the determination of the likelihood of FH based on the formal diagnostic criteria.
History of premature CAD or other CVD
- Angina pectoris
- Myocardial infarction
- Peripheral vascular diseases
Note: Although stroke is possible, it is less common in FH than premature CAD [Huxley et al 2003].
Physical examination findings
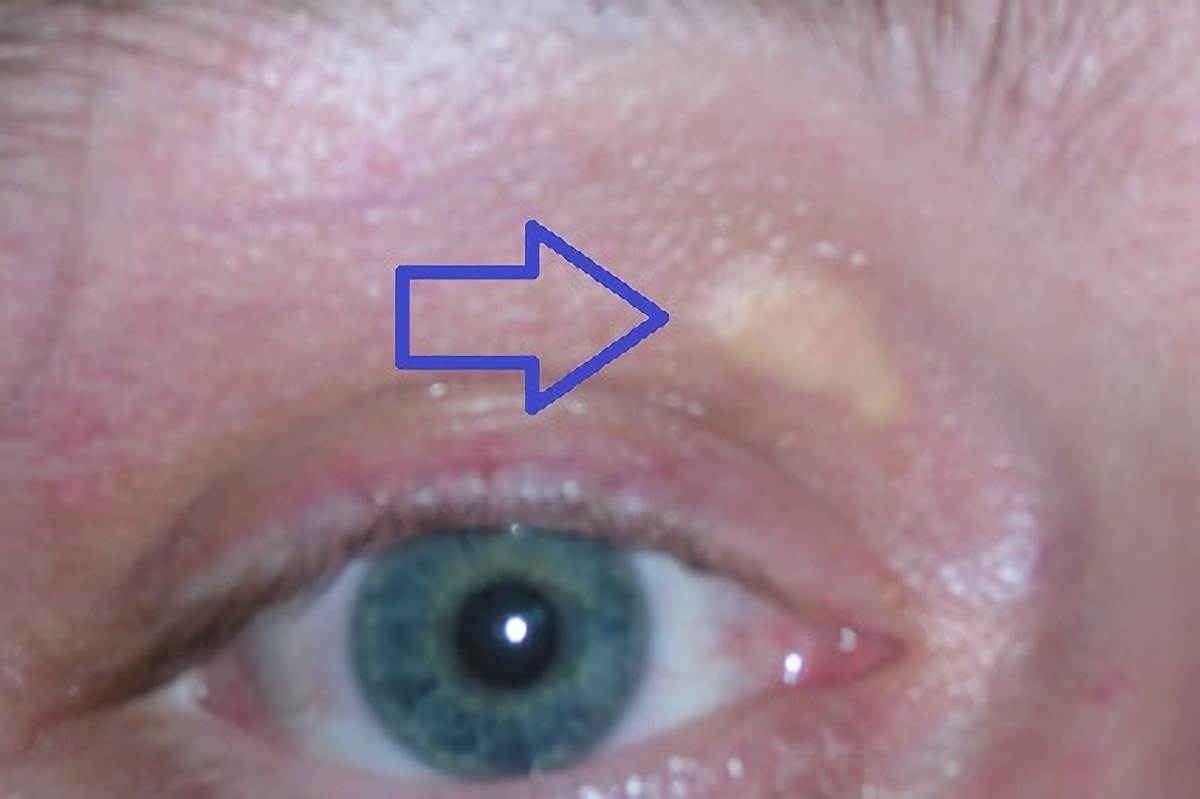
- Xanthomas (patches of yellowish cholesterol buildup). Common locations include around the eyelids, tendons of the elbows, hands, knees, and feet, particularly the Achilles tendon. Interdigital xanthomas occur in individuals with homozygous FH.
- Corneal arcus (white, gray, or blue opaque ring in the corneal margin). Because this becomes increasingly common in the general population with age, it is only diagnostic in younger individuals, particularly before age 45 years.
Note: As statin treatment has become more common, it is possible that individuals have fewer visible signs of FH, complicating the application of the diagnostic criteria.
Family history of any of the following
- Familial hypercholesterolemia
- High levels of LDL-C
- Early-onset (i.e., age <50 years) CAD (especially myocardial infarction)
- Xanthomas
Identification of a pathogenic variant in a gene known to be associated with FH (see Table 1) is the gold standard for diagnosis accepted in many countries [Nordestgaard et al 2013].
- The likelihood of identifying a pathogenic variant by molecular testing increases with higher LDL-C levels.
- In the United States, genetic testing is recommended when other laboratory tests have not definitively established the diagnosis of FH.
Molecular genetic testing approaches can include serial single-gene testing and use of a multigene panel:
- Serial single-gene testing can be considered. Sequence analysis of LDLR is performed first and followed by LDLR deletion/duplication analysis if no pathogenic variant is found. Sequence analysis of APOB and PCSK9 can be performed next if no pathogenic variant is found.
- A multigene panel that includes APOB, LDLR, PCSK9, and other genes of interest (see Differential Diagnosis) may also be considered. Note: (1) The genes included and the sensitivity of multigene panels vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview; thus, clinicians need to determine which multigene panel is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.For an introduction to multigene panels click here. More detailed information for clinicians ordering genetic tests can be found here.
Table 1.
Molecular Genetic Testing Used in Familial Hypercholesterolemia (FH)
| Gene 1 | Proportion of FH Attributed to Pathogenic Variants in This Gene 2 | Proportion of Pathogenic Variants 3 Detectable by Method | |
|---|---|---|---|
| Sequence analysis 4 | Gene-targeted deletion/duplication analysis 5 | ||
| APOB | 1%-5% | >99% | 1 individual 6 |
| LDLR | 60%-80% | >90% 7 | ~2.5%-10% 8 |
| PCSK9 | 0%-3% | ~100% | None reported 9 |
| Unknown 10, 11 | 20%-40% | NA | |
- 1.
See Table A. Genes and Databases for chromosome locus and protein.
- 2.
The yield for genetic testing varies by the pre-test probability of the disease as determined by the clinical diagnostic criteria. In "definite" FH the yield of genetic testing for identification of a pathogenic variant approaches 95%; in "probable" or "possible" FH the yield is lower (~70%) [Motazacker et al 2012, Awan et al 2013].
- 3.
See Molecular Genetics for information on allelic variants detected in this gene.
- 4.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 5.
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications.
- 6.
Huang et al [1989]
- 7.
The majority of pathogenic variants in LDLR are detectable by sequence analysis, including those in the regulatory region (the majority occurring within 200 bp upstream of the initiation codon) if that region is targeted for sequencing [Dedoussis et al 2004].
- 8.
Horsthemke et al [1987], Dedoussis et al [2004], Bertolini et al [2013]
- 9.
No deletions or duplications involving PCSK9 have been reported to cause familial hypercholesterolemia [De Castro-Orós et al 2010].
- 10.
De Castro-Orós et al [2010]
- 11.
It has been suggested that a polygenic etiology is most likely in the majority of individuals with a clinical diagnosis of FH in whom no pathogenic variant in one of the three known genes can be identified. This suggestion is based on the presence in these individuals of a greater than average number of common LDL-C-raising variants (i.e., a high "LDL-SNP score") [Talmud et al 2013].
Clinical Characteristics
Clinical Description
Familial Hypercholesterolemia (FH)
Coronary artery disease (CAD) and cardiovascular disease (CVD). Elevated LDL cholesterol (LDL-C) leads to atherosclerotic plaque deposition in the coronary arteries and proximal aorta at an early age, leading over time to an increased risk for cardiovascular disease (CVD), which by definition includes coronary artery disease (CAD) (manifest as angina and myocardial infarction) and stroke [Scientific Steering Committee 1991, Versmissen et al 2008, Elis et al 2011, Raal & Santos 2012]. In FH, CAD is the more common cardiovascular disease; stroke occurs more rarely and some evidence suggests that the risk for stroke may not be greater than in the general population [Huxley et al 2003].
All individuals with FH should be considered "high risk" (i.e., increased ~20-fold) for CAD. Recent data suggest that individuals with an LDL-C >190 mg/dL (>4.9 mmol/L) and a pathogenic variant in one of the genes listed in Table 1 have a 22-fold increased risk for CAD while those without a pathogenic variant have a sixfold increased risk for CAD over the general population [Khera et al 2016].
Natural history studies from the pre-statin era suggest that untreated men are at 50% risk for a fatal or nonfatal coronary event by age 50 years; untreated women are at 30% risk by age 60 years [Slack 1969, Stone et al 1974, Civeira 2004, Goldberg et al 2011, Reiner et al 2011]. See Figure 1. Of note, standard Framingham or other risk classification schemes are not applicable to persons with FH [Goldberg et al 2011, Reiner et al 2011, Nordestgaard et al 2013].

Figure 1.
LDL cholesterol burden in individuals with or without familial hypercholesterolemia as a function of the age of initiation of statin therapy Data derived from Starr et al [2008] and Huijgen et al [2012]. Figure from Nordestgaard et al [2013]; used by (more...)
Lipid-lowering therapy with statin-based regimens (see Management, Treatment of Manifestations) significantly increases survival [Nordestgaard et al 2013, Vuorio et al 2013] and reduces morbidity [Versmissen et al 2008, Elis et al 2011]. Additional medications such as ezetimibe, bile acid binding resins, or PCSK9 inhibiters are often necessary to achieve optimal LDL-C reduction [Gidding et al 2015].
Data from the CASCADE-FH registry reveals that upwards of 61% of adults with FH have at least one modifiable cardiovascular risk factor. Coronary heart disease was reported in 47% of men with FH, with a median age of onset of 47 years. Coronary heart disease was reported in 29% of women with FH, with a median age of onset of 55 years. A high-dose statin was being taken by 42% of individuals with FH, 33% of individuals were on a low-dose statin, and 25% of individuals were not taking a statin. The most common reasons for lack of statin use included intolerance or allergy (60%), patient preference (11%), physician preference (11%), pregnancy (3%), cost (1%), and clinical trial participation (1%). Twenty-five percent of treated individuals had an LDL-C below 100 mg/dL and 41% of individuals achieved a 50% or greater reduction in LDL-C from untreated values [deGoma et al 2016].
Xanthomas are patches of yellowish cholesterol buildup in particular areas of the body as a result of extremely high levels of LDL-C. Xanthomas may worsen with age in persons who are not treated. In persons treated with LDL-C-lowering therapy, the xanthomas can become smaller. Common locations:
- Xanthomas often occur around the eyelids (xanthelasma palpebrarum or more commonly just xanthelasma) [Dey et al 2013].
- Tendonous xanthomas can occur in the elbows, hands, knees, and feet, particularly the Achilles tendon [Tsouli et al 2005, Elis et al 2011]. These are historically described in 30%-50% of persons with FH, although more recent studies show a lower prevalence likely due to widespread statin use [Perez de Isla et al 2016].
- Interdigital xanthomas (between the fingers) occur in individuals with homozygous FH.
Corneal arcus (white, gray, or blue opaque ring in the corneal margin). Because corneal arcus becomes increasingly common in the general population with age, it is only diagnostic in younger individuals, particularly before age 45 years.
Homozygous Familial Hypercholesterolemia (HoFH)
Homozygous familial hypercholesterolemia refers to familial hypercholesterolemia resulting from biallelic (homozygous or compound heterozygous) pathogenic variants in one of the genes listed in Table 1 (APOB, LDLR, and PCSK9). The prevalence of HoFH, estimated at 1:160,000 to 1:250,000, is much lower than FH [Nordestgaard et al 2013, Cuchel et al 2014].
In adults with HoFH, untreated LDL-C levels are generally, but not always, >500 mg/dL (>13 mmol/L). Levels can be lower in children or in treated individuals. Thus, LDL-C levels are not sufficient to confirm a diagnosis [Cuchel et al 2014].
In addition to xanthomas around the eyelids and tendonous xanthomas, individuals with HoFH can have interdigital xanthomas (between the fingers).
The natural history of HoFH differs from that of FH. Most individuals with HoFH experience severe CAD by their mid-20s. The rate of either death or coronary bypass surgery by the teenage years is high [Raal & Santos 2012]. Severe aortic stenosis is also common [Raal & Santos 2012, Raal et al 2016a, Raal et al 2016b].
Statins are often relatively ineffective in the treatment of HoFH because their efficacy largely depends on the upregulation of functional LDL receptors in the liver. In individuals with HoFH, activity of both copies of the LDL receptor are absent or greatly reduced [Raal & Santos 2012]; therefore, therapy for HoFH often requires LDL apheresis in addition to the use of multiple other medications (see Management, Treatment of Manifestations) [Raal et al 2010, Raal 2013, Cuchel et al 2014].
Phenotype Correlations by Gene
APOB. FH caused by a heterozygous APOB pathogenic variant is reported to be less severe than FH caused by a heterozygous pathogenic variant in LDLR or PCSK9 [Hopkins et al 2011].
Genotype-Phenotype Correlations
LDLR. Complete loss-of-function variants in LDLR generally lead to more severe disease due to higher LDL-C levels [Khera et al 2016]. Partial loss-of-function variants in LDLR result in less severe disease due to lower LDL-C levels.
Penetrance
APOB. Penetrance for FH can be incomplete in persons with a heterozygous APOB pathogenic variant [Fahed & Nemer 2011].
LDLR. Recent findings suggest that only 73% of individuals with a heterozygous LDLR pathogenic variant have an LDL level >130 mg/dL, suggesting lower penetrance than previously proposed [Khera et al 2016].
PCSK9
- Penetrance is approximately 90% in persons heterozygous for the c.381T>A (p.Ser127Arg) pathogenic variant.
- Penetrance in persons heterozygous for the p.Asp374Tyr pathogenic variant is high, with FH manifesting at a young age [Naoumova et al 2005].
- Penetrance for other heterozygous PCSK9 pathogenic variants remains largely unknown [Cariou et al 2011].
Prevalence
The prevalence of FH in the general population has traditionally been cited as 1:500. However, emerging data suggest that the prevalence of FH is higher in white/European populations, perhaps as common as 1:200 [Benn et al 2012, Nordestgaard et al 2013].
FH is more common (due to founder effects) in several populations (Table 2). Of note, data are limited on prevalence of FH in most African and South Asian/Indian populations.
Table 2.
Prevalence of FH in Select Populations
| Population | Prevalence |
|---|---|
| General population | 1:250 |
| French Canadian 1 | 1:270 |
| Old Order Amish 2 | 1:10 |
| Christian Lebanese | 1:85 |
| Tunisia | 1:165 |
| South African Afrikaners | 1:72 to 1:100 |
| South African Ashkenazi Jews | 1:67 |
Austin et al [2004]
- 1.
A common >15-kb (60% of alleles) and an ~5-kb (5% of alleles) LDLR deletion results in a significant increase in LDL-C [Simard et al 2004].
- 2.
Amish individuals heterozygous for APOB p.Arg3500Gln (the most common pathogenic variant in the Amish community) have average LDL-C levels below the suggested minimum for a diagnosis of FH. However, coronary artery calcification and atherosclerosis still occurred in heterozygous Amish individuals with lower average LDL-C levels [Andersen et al 2016].
Differential Diagnosis
Conditions with clinical findings similar to those of familial hypercholesterolemia (FH) include the following:
- 27-hydroxylase deficiency (cerebrotendonous xanthomatosis), which is characterized by xanthomas. Distinguishing features are normal LDL cholesterol (LDL-C) levels and the presence of dementia, ataxia, and cataracts. Biallelic pathogenic variants in CYP27A1 are causative; inheritance is autosomal recessive.
- Hyperlipoproteinemia type III (familial dysbetalipoproteinemia) (OMIM 107741). Clinical features may include xanthomas. Hyperlipoproteinemia type III is caused by biallelic pathogenic variants in APOE. Inheritance is autosomal recessive [Fung et al 2011].
- Sitosterolemia (phytosterolemia), which is distinguished by normal or only mildly elevated LDL-C levels. Biallelic pathogenic variants in either ABCG5 or ABCG8 are causative; inheritance is autosomal recessive.
- Polygenic hypercholesterolemia. Talmud et al [2013] found that individuals with features suggestive of FH in whom an LDLR, PCSK9, or APOB pathogenic variant was not identified are in fact likely to have polygenic hypercholesterolemia. In this case, individuals have a high genetic risk score for more common LDL-C raising alleles.
- Extremely elevated lipoprotein a (Lpa) (OMIM 152200). Individuals with very high Lpa often have a personal and family history of early-onset CAD and very elevated LDL-C levels [Langsted et al 2016]. High Lpa levels are also widely appreciated to synergistically increase risk in individuals with FH. The disorder is inherited in an autosomal dominant manner and caused by variants in the number of kringle IV type 2 repeats in LPL.
Conditions with laboratory findings similar to those of FH include the following [Goldberg et al 2011]:
- Hypercholesterolemia secondary to obesity, diabetes mellitus, hypothyroidism, drugs (e.g., steroids), or kidney disease. Inheritance follows a non-Mendelian pattern.
- Autosomal recessive hypercholesterolemia (OMIM 603813) caused by biallelic pathogenic variants in LDLRAP1. Persons with biallelic pathogenic variants have LDL-C >400 mg/dL (>10 mmol/L), whereas heterozygotes have normal LDL-C levels.
- Familial combined hyperlipidemia (FCHL) (OMIM 144250), associated with elevated LDL-C and triglycerides. FCHL is genetically heterogeneous. Only 10%-20% of individuals show elevated levels in childhood (usually in the form of hypertriglyceridemia). FCHL is inherited in an autosomal dominant manner and caused by pathogenic variants in LPL [Brahm & Hegele 2016]. Additional loci have been mapped to APOB and USF1 in a minority of families [Naukkarinen et al 2006, Hegele et al 2009].
Management
Evaluations Following Initial Diagnosis
To establish the extent of disease and needs of an individual diagnosed with familial hypercholesterolemia (FH) the following evaluations are recommended in adults and children:
- Measurement of pre-treatment lipid values and lipoprotein(a) levels when possible
- Exclusion of concurrent illnesses (kidney disease, acute myocardial infarction, infection) that can affect lipid values
- Lipid panel including total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides
- Consultation with a lipid specialist or clinician with expertise in FH
- Recommended in some guidelines: noninvasive imaging modalities in children (e.g., measurement of carotid intima-media thickness) to help inform treatment decisions [Martin et al 2013]
- Consultation with a clinical geneticist and/or genetic counselor
Treatment of Manifestations
Adults with FH
All individuals with FH should be considered "high risk" for coronary artery disease (CAD) and should be treated actively to lower cholesterol levels. Note that standard Framingham or other risk classification schemes are not applicable [Goldberg et al 2011, Hopkins et al 2011, Stone et al 2014]. The most current recommendations (summarized here) for the management of FH used in the United States are from the American Heart Association [Gidding et al 2015] (full text) and largely reflect earlier recommendations from the National Lipid Association (NLA).
For adults, treatment should begin as soon as possible after diagnosis. All adults with FH require diet/lifestyle management, and almost without exception will also require cholesterol-lowering drug therapy.
- Risk factors (e.g., smoking, diabetes mellitus, hypertension) are the same in FH as in the general population; aggressive management is required to reduce CAD risk, with special attention to smoking cessation.
- Regular physical activity, a healthy diet (reduce saturated fat intake, increase intake of soluble fiber to 10-20 g/day), and weight control should be emphasized.
- Blood pressure should be treated to 140/90mm Hg (or 130/80 mm Hg in those with diabetes mellitus).
- Low-dose aspirin (75-81 mg/day) should be considered in those at high risk for CAD or stroke.
- Consider referral to a lipid specialist with expertise in FH if LDL-C concentrations are not reduced with maximal medical therapy. (Note: Although it has not been specified, this recommendation generally pertains to LDL-C levels that cannot be reduced by ≥50% with maximal medical therapy over a 6-month period.)
- Testing of first-degree and second-degree relatives should be recommended to all individuals with FH (see Evaluation of Relatives at Risk).
- For adults with FH age 20 years or older, treatment with statins should be initiated to reduce the LDL-C level ≥50% or to <100 mg/dL (<2.6 mmol/L) [Hopkins et al 2011, Nordestgaard et al 2013]. Many guidelines suggest a target LDL-C of <100 mg/dL even in those without known CAD as individuals with FH have had a lifelong burden of high LDL-C [Gidding et al 2015].
For persons with FH with any of the following CAD risk factors, drug treatment may need to be intensified (see *Note) to achieve more aggressive treatment goals (LDL-C <100 mg/dL [<2.6 mmol/L] and non-HDL-C <130 mg/dL [<3.4 mmol/L]). Risk factors:
- Clinically evident CAD or other atherosclerotic cardiovascular disease; the goal is LDL-C level of <70 mg/dL (<1.8 mmol/L)
- Diabetes mellitus or metabolic syndrome
- Family history of very early CAD (men age <45 years; women age <55 years)
- Current smoking
- High lipoprotein(a) (≥50 mg/dL [≥1.3 mmol/L] using an isoform insensitive assay)
The second-line agent for individuals with FH who do not achieve acceptable LDL-C levels is generally ezetimibe. Treatment options for intensification of therapy after ezetimibe or for those intolerant of statins include bile acid sequestrants and/or PCSK9 inhibitors. Although niacin has been used as an adjunctive therapy in individuals with FH, given recent data, niacin is generally not favored before the other options have been exhausted [Guyton et al 2013, FDA 2016] (see Table 3).
In persons with FH without any of the CAD risk factors listed above, intensification of drug therapy (see *Note) should be strongly considered if 50% reduction in LDL-C is not achieved after six months on maximum statin therapy. For adults, some guidelines call for intensification of treatment if the goal LDL-C of <100 mg/dL (<2.6 mmol/L) is not achieved [Nordestgaard et al 2013].
*Note: (1) The lipid-lowering therapy should initially be statin-based with titration of doses every few months in order to use the highest tolerated dose of a potent statin, followed by addition of other drugs if the targeted LDL-C level is not achieved. (2) The potential benefit of multidrug regimens should be weighed against the increased cost and potential for adverse effects and decreased adherence.
Table 3.
Current Recommended Drug Therapies for Adults with FH
| Class | Primary (1O) and Secondary (2O) Mechanism of Action | LDL-Lowering Response |
|---|---|---|
| Statins | ↑ LDLR activity (1O) | >35% 1, 2 |
| Cholesterol absorption inhibitors (ezetimibe) | ↓ Cholesterol absorption (1O) ↑ LDLR activity (2O) | 15% 1, 3 |
| Mipomersen (APOB antisense) 4 | Blocks APOB production in the liver | 50% 5 |
| MTP inhibitor (lomitapide) 4 | ↓ microsomal triglyceride transfer protein activity (1O) Inhibition of LDL production (2O) | 50% 5 |
| PCSK9 inhibitors (alirocumab, evolocumab) | ↓ LDL-receptor degradation | 50% 6 |
| Bile acid sequestrants (cholestyramine, colesevelam) | ↓ Bile acid reabsorption (1O) ↑ LDLR activity (2O) | 15% 1, 3 |
| Stanol esters | ↓ Cholesterol absorption (1O) ↑ LDLR activity (2O) | 10% 1, 3 |
Some guidelines call for the addition of n-3 polyunsaturated fatty acids or fibrates if triglycerides remain elevated after the LDL-C is controlled.
- 1.
Often ineffective in HoFH
- 2.
Kastelein et al [2008]
- 3.
Rader et al [2003]
- 4.
Approved only for adults with HoFH
- 5.
Cuchel et al [2014]
- 6.
Raal & Santos [2012]
Children with FH
Guidelines for the management of children and individuals up to age 21 years have been published by the National Heart, Lung, and Blood Institute (full text). Children should be considered for drug treatment with statin-based regimens when:
- LDL-C levels are ≥190 mg/dL (≥4.9 mmol/L).
- LDL-C levels are ≥160 mg/dL (≥4.1 mmol/L) and at least two other risk factors are present.
US-based guidelines from the NLA (full text):
- Consultation or referral to a lipid specialist is recommended.
- Management of diet and physical activity is recommended at an early age.
- Statins are the preferred initial pharmacologic treatment in children. Consideration should be given to starting statin treatment at age eight years or older. In special cases, such as children with homozygous FH, drug treatment needs to be initiated prior to age eight years.
- The goal of lipid-lowering therapy in children with FH is a ≥50% reduction in LDL-C or LDL-C <130 mg/dL (<3.4 mmol/L). Note: More aggressive lowering of LDL-C levels should be considered for children with additional CAD risk factors (e.g., family history of CAD, high blood pressure, unhealthy diet or exercise behaviors, obesity).
Children and Adults with Homozygous FH (HoFH)
NLA guidelines for homozygous FH (full text):
- Referral to a lipid specialist is indicated.
- Early initiation of therapy and monitoring are recommended.
- Multiple drug therapy is usually needed. Several different classes of medications are currently being used to treat HoFH (see Table 3).
- Since many cholesterol-lowering medications target the LDL receptor, effectiveness in persons with FH with biallelic loss-of function LDLR pathogenic variants can be limited [Cuchel et al 2014]. Statins are often relatively ineffective in the treatment of HoFH because their efficacy largely depends on the upregulation of functional LDL receptors in the liver. In HoFH, both copies of the LDL receptor have absent or greatly reduced activity [Raal & Santos 2012].
- High-dose statins, ezetimibe, and bile-acid binding resins may be effective in some persons with HoFH, especially those with some residual LDLR activity.
- For HoFH, evolocumab is a PCSK9 inhibitor that showed a 40% mean reduction in LDL-C compared with placebo; however, individuals with two loss-of-function variants saw no response [Raal et al 2015]. PCSK9 inhibitors have not been formally approved in children with FH.
- HoFH-specific medications (lomitapide and mipomersen) are effective even with complete loss of LDL receptor function and – though not formally FDA approved for children – should strongly be considered.
- Despite these options, many individuals with HoFH (especially those with complete loss of LDL receptor function) will require ongoing LDL apheresis. LDL apheresis (≤2x/week) is often required starting from a young age. Apheresis can lower LDL-C levels by 80% acutely and 30% chronically (weekly or biweekly). Apheresis is offered at a limited number (~40-50) of centers in the United States; many states do not have an apheresis center.
- Liver transplantation is also being used in rare circumstances in some centers [Martinez et al 2016]
Prevention of Primary Manifestations
Preventive measures include the following:
- Statin-based therapy with addition of other medications as needed
- Reduced intake of saturated fat
- Increased intake of soluble fiber to 10-20 g/day
- Increased physical activity
- Not smoking
Surveillance
Children. Guidelines for the management of children have been published by multiple national and international organizations [Daniels et al 2008, DeMott et al 2008, Descamps et al 2011, Martin et al 2013] (see Published Guidelines / Consensus Statements).
A child who has a family history of FH or of premature CAD, who is heterozygous for the FH pathogenic variant in his or her family, or who has an elevated serum cholesterol concentration should have lipid levels checked starting as early as age two years [Goldberg et al 2011]. It is reasonable to check a non-fasting lipid level first and, if borderline, to follow with measurement of LDL-C. Some guidelines state that elevation of two consecutive measures of LDL-C are needed to confirm a diagnosis of FH [Martin et al 2013].
An LDL-C level of >130 mg/dL (>3.4 mmol/L) in a child is suspicious for FH and an LDL of >160 mg/dL (>4.1 mmol/L) is relatively specific for FH.
During treatment, individuals of any age with:
- FH should have lipid levels monitored as recommended;
- HoFH should be monitored with various imaging modalities (including echocardiogram, CT angiogram, and cardiac catheterization) as recommended [Raal & Santos 2012].
Agents/Circumstances to Avoid
The following should be avoided:
- Smoking
- High intake of saturated and trans unsaturated fat
- Excessive intake of cholesterol
- Sedentary lifestyle
- Obesity
- Hypertension
- Diabetes mellitus
Evaluation of Relatives at Risk
The CDC has classified FH as a Tier 1 condition indicating a significant benefit from performing family-based cascade screening using cholesterol testing with or without DNA analysis on relatives of affected persons with FH in order to identify previously unknown cases of FH and provide those people with lifesaving treatment. Early diagnosis and treatment of relatives at risk for FH can reduce morbidity and mortality [Goldberg et al 2011, Ned & Sijbrands 2011, Reiner et al 2011].
The genetic status of at-risk family members can be clarified by EITHER of the following:
- Molecular genetic testing if the pathogenic variant has been identified in an affected family member
- Measurement of LDL-C level. Table 4 provides age-specific total cholesterol and LDL-C levels [Williams et al 1993]. Note: Age-specific LDL cut-offs are also available based on more contemporary data from the United Kingdom [Starr et al 2008].
Table 4.
Total and LDL Cholesterol Levels in Heterozygotes for FH Based on Degree of Relatedness to an Individual with FH
| Degree of Relatedness to an Affected Individual | First Degree 1 | Second Degree 2 | Third Degree 3 | |
|---|---|---|---|---|
| Cholesterol Levels | Total Cholesterol (LDL Cholesterol) in mg/dL | |||
| Age | <20 | 220 (155) | 230 (165) | 240 (170) |
| 20-29 | 240 (170) | 250 (180) | 260 (185) | |
| 30-39 | 270 (190) | 280 (200) | 290 (210) | |
| 40+ | 290 (205) | 300 (215) | 310 (225) | |
- 1.
A parent, sib, or child. First-degree relatives share about half of their genes.
- 2.
An uncle, aunt, nephew, niece, grandparent, grandchild, or half-sib. Second-degree relatives share about one quarter of their genes.
- 3.
A first cousin, great-grandparent, or great-grandchild. Third-degree relatives share about one eighth of their genes.
Of note, all national and international guidelines for FH call for "cascade testing" of relatives at risk (i.e., systematic testing of first- and second-degree relatives of the index case [proband]). Evidence supports the use of genetic testing in cascade testing algorithms to improve the detection of FH [DeMott et al 2008, Wierzbicki et al 2008, Wald et al 2016].
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Pregnancy Management
Statins are contraindicated during pregnancy; women with FH who are considering a pregnancy should be counseled of this risk and statins should be discontinued prior to conception.
Pregnant women should incorporate all the other recommended lifestyle changes including low saturated and trans unsaturated fat intake, no smoking, and high dietary soluble fiber intake (see Agents/Circumstances to Avoid).
Pharmacologic treatment during pregnancy
- Statins are contraindicated in pregnancy due to concerns for teratogenicity. The use of statins during human pregnancy has not definitively been associated with adverse fetal outcome; however, the role of cholesterol in embryologic development has led to theoretic concerns about the effect of these medications on a developing fetus and a recommendation that alternative medications be considered during pregnancy and lactation. Nursing mothers should not take statins.
- Bile acid binding resins (colesevelam, cholestyramine) are generally considered safe (Class B for pregnancy). Based primarily on animal studies, cholestyramine use during pregnancy has not been associated with an increased risk of fetal anomalies. However, use of cholestyramine could theoretically cause depletion of maternal fat-soluble vitamins, including vitamin K.
- LDL apheresis is also occasionally used.
- Regarding other agents:
- PCSK9 inhibitors. Use during pregnancy has not been well studied.
- Ezetimibe. Use during human pregnancy has not been well studied.
- Niacin. The use of pharmacologic doses of niacin, an essential vitamin, has not been studied in human pregnancy. The recommended upper limit of niacin intake during pregnancy is 30-35 mgs/day; higher doses have been associated with toxicity.
Therapies Under Investigation
CAD outcome trials of PCSK9 inhibitors are currently underway.
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for access to information on clinical studies for a wide range of diseases and conditions.