Short-Rib Thoracic Dysplasia 1 With Or Without Polydactyly
Description
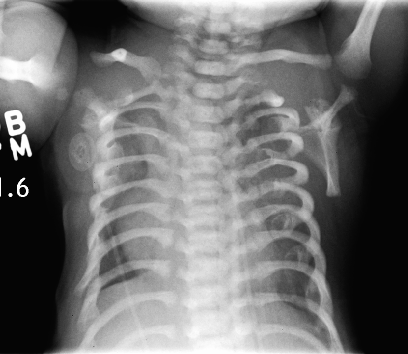
Short-rib thoracic dysplasia (SRTD) with or without polydactyly refers to a group of autosomal recessive skeletal ciliopathies that are characterized by a constricted thoracic cage, short ribs, shortened tubular bones, and a 'trident' appearance of the acetabular roof. SRTD encompasses Ellis-van Creveld syndrome (EVC) and the disorders previously designated as Jeune syndrome or asphyxiating thoracic dystrophy (ATD), short rib-polydactyly syndrome (SRPS), and Mainzer-Saldino syndrome (MZSDS). Polydactyly is variably present, and there is phenotypic overlap in the various forms of SRTDs, which differ by visceral malformation and metaphyseal appearance. Nonskeletal involvement can include cleft lip/palate as well as anomalies of major organs such as the brain, eye, heart, kidneys, liver, pancreas, intestines, and genitalia. Some forms of SRTD are lethal in the neonatal period due to respiratory insufficiency secondary to a severely restricted thoracic cage, whereas others are compatible with life (summary by Huber and Cormier-Daire, 2012 and Schmidts et al., 2013).
There is phenotypic overlap with the cranioectodermal dysplasias (Sensenbrenner syndrome; see CED1, 218330).
Genetic Heterogeneity of Asphyxiating Thoracic Dysplasia
SRTD1 has been mapped to chromosome 15q13. See also SRTD2 (611263), caused by mutation in the IFT80 gene (611177); SRTD3 (613091), caused by mutation in the DYNC2H1 gene (603297); SRTD4 (613819), caused by mutation in the TTC21B gene (612014); SRTD5 (614376), caused by mutation in the WDR19 gene (608151); SRTD6 (263520), caused by mutation in the NEK1 gene (604588); SRTD7 (614091), caused by mutation in the WDR35 gene (613602); SRTD8 (615503), caused by mutation in the WDR60 gene (615462); SRTD9 (266920), caused by mutation in the IFT140 gene (614620); SRTD10 (615630), caused by mutation in the IFT172 gene (607386); SRTD11 (615633), caused by mutation in the WDR34 gene (613363); SRTD13 (616300), caused by mutation in the CEP120 gene (613446); SRTD14 (616546), caused by mutation in the KIAA0586 gene (610178); SRTD15 (617088), caused by mutation in the DYNC2LI1 gene (617083); SRTD16 (617102), caused by mutation in the IFT52 gene (617094); SRTD17 (617405), caused by mutation in the TCTEX1D2 gene (617353); SRTD18 (617866), caused by mutation in the IFT43 gene (614068); SRTD19 (617895), caused by mutation in the IFT81 gene (605489); and SRTD20 (617925), caused by mutation in the INTU gene (610621).
See also SRTD12 (Beemer-Langer syndrome; 269860).
Clinical FeaturesMaroteaux and Savart (1964) described asphyxiating thoracic dystrophy and noted that the skeletal changes in the rib cage, pelvis, and limbs were similar to those observed in Ellis-van Creveld syndrome (EVC; 225500). Pirnar and Neuhauser (1966) reported 3 affected brothers and noted the presence of polydactyly without dysplasia of the fingernails. Those who survived early childhood tended to develop other disorders, including chronic nephritis (Wahlers, 1966) and intestinal malabsorption (Karjoo et al., 1973).
Hanissian et al. (1967) reported 2 families, each with 2 affected brothers; 1 family was of African descent. These authors thought that the family reported by Shapira et al. (1965) had this condition.
Langer (1968) pointed out that in those cases with polydactyly, differentiation from Ellis-van Creveld syndrome may not be possible on radiologic grounds alone. Polydactyly is an inconstant feature of ATD and, when present, usually also affects the feet. In contrast, polydactyly of the hands is a constant feature in EVC, but the feet are uncommonly affected. The main visceral abnormality in ATD is renal, whereas it is cardiac in EVC.
Shokeir (1970) described 5 related affected persons of Norwegian extraction with asphyxiating thoracic dystrophy. Cystic renal changes (Potter type IV) were described. Cystic lesions may occur in the kidney, liver, and pancreas (Hopper et al., 1979; Landing et al., 1980).
Finegold et al. (1971) reported a case with hypoplastic lungs and a marked reduction in the number of alveoli at autopsy.
Oberklaid et al. (1977) reported 10 cases. Renal and hepatic changes were progressive, and renal failure was the cause of death in at least 2 patients. One remarkable case was that of a boy who was still alive at age 15 years and at the 25th percentile for height. He had a small chest, but short ribs were the only radiologic finding. A 32-year-old patient was reported by Friedman et al. (1975).
Turkel et al. (1985) studied 7 neonatal cases at autopsy; 2 were sibs born of consanguineous parents. Dwarfing was not pronounced; the limbs were short in only one infant who also had polydactyly. Enchondral ossification was irregular in sections of femur, vertebra, and rib. Pulmonary hypoplasia was associated with the small thorax. Periportal fibrosis, bile duct proliferation, cirrhosis (in 1 case), and variable pancreatic fibrosis were also described.
Whitley et al. (1987) described liver dysfunction associated with direct hyperbilirubinemia and hepatic fibrosis in the newborn period. Hudgins et al. (1990) described 2 sibs with this disorder who had progressive hepatic dysfunction associated with cirrhosis. Giorgi et al. (1990) described 2 sisters with a mild form of the syndrome.
Zack and Beighton (1995) described what they designated spondyloenchondromatosis (see 607944) in 1 of 6 children of a consanguineous couple of mixed ancestry. When first seen at the age of 2.5 years, a tentative diagnosis of pseudoachondroplasia (177170) had been made but the features later evolved to a radiologic appearance diagnostic of spondyloenchondromatosis. With hindsight, the configuration of the pelvis at age 2.5 years was somewhat suggestive of asphyxiating thoracic dysplasia. Subsequently, marked constriction of the chest developed, as indicated by the photographs taken at the age of 13 years.
Labrune et al. (1999) reported 3 children with Jeune syndrome who had clinical and laboratory evidence of liver disease. The liver involvement was severe and led to hepatic fibrosis and later to biliary cirrhosis with portal hypertension. In 1 patient, prolonged neonatal cholestasis was the initial manifestation, whereas in the other 2, hepatic lesions were recognized late when fibrosis or even cirrhosis had developed. Treatment with ursodeoxycholic acid appeared to control the progression of hepatic dysfunction, based on improvement in clinical and laboratory data. The authors suggested that hepatic function should be followed regularly in patients with Jeune syndrome, including measurements of serum biliary acid concentration.
Kajantie et al. (2001) described 3 sibs with ATD whose neonatal symptoms ranged from mild respiratory distress to asphyxia and death. The authors reported difficulties in the prenatal diagnosis of the younger sibs prior to the third trimester. They proposed that even severely affected patients may have a favorable prognosis given new neonatal intensive care treatment options.
Other FeaturesRetinal degeneration resembling Leber congenital amaurosis (104000) was described by Allen et al. (1979), Bard et al. (1978), and Phillips et al. (1980). Wilson et al. (1987) described the progressive electroretinographic abnormalities in an affected brother and sister.
Pancreatic cysts were reported by Hopper et al. (1979).
Singh et al. (1988) described 4 patients, including 2 sibs, with Jeune syndrome and mild congenital hydrocephalus. All 4 were males; 3 had postaxial polydactyly.
Rinaldi et al. (1990) reported 2 sisters who had both Jeune syndrome and cystinuria (220100). The parents, living in Italy, were presumably unrelated. The possibility of linkage of the 2 genes was considered.
Lehman et al. (2010) reported 2 sibs, born of consanguineous Filipino parents, with a combination of ATD and Joubert syndrome (213300). Features included developmental delay, hypotonia, molar tooth sign on brain MRI, small thorax, short limbs and ribs, progressive renal failure, bile duct dilatation, oculomotor apraxia, and retinal dystrophy in 1. Lehman et al. (2010) described 2 additional unrelated patients with similar features of both Joubert syndrome and ATD, although without renal or hepatic involvement. The clinical observation of cooccurrence of ATD and Joubert syndrome in these patients suggested the involvement of a single causative ciliary gene required for both skeletal and neurologic development.
InheritanceShokeir et al. (1971) presented strong evidence for recessive inheritance of ATD in a Norwegian kindred, and raised the possibility that chest deformity may be a manifestation of the gene in the heterozygote.
Tuysuz et al. (2009) reported 10 patients with Jeune syndrome, all of whom were born of consanguineous parents.
Clinical ManagementBarnes et al. (1971) reported successful thoracic reconstruction in a child whose sib had died of the disorder and whose mother was thought to have been affected (Barnes et al., 1969). (This family was later thought (Burn et al., 1986) to have a 'new' disorder called Barnes syndrome; see 187760.)
Takada et al. (1994) reported surgical thoracic expansion according to the procedure of Todd et al. (1986) in a 15-month-old girl requiring mechanical ventilation for asphyxiating thoracic dystrophy. At the age of 4 years, she was free from respiratory distress, was of normal intelligence, and was able to lead an active life.
MappingMorgan et al. (2003) performed a genomewide linkage search using autozygosity mapping in 4 consanguineous families with ATD: 3 from Pakistan and 1 from southern Italy. In these families, as well as in a fifth consanguineous family from France, they localized a novel ATD locus (ATD1) to 15q13 with a maximum cumulative 2-point lod score of 3.77 at theta = 0.00 for marker D15S1031. Investigation of a further 4 European kindreds with no known parental consanguinity showed evidence of marker homozygosity across a similar interval of 1.2 cM on chromosome 15. Families with both mild and severe forms of ATD mapped to 15q13, but mutation analysis of 2 positional candidate genes, gremlin (GREM1; 603054) and formin (FMN1; 136535), did not show pathogenic mutations.
By homozygosity mapping of 2 sibs, born of consanguineous parents, with a phenotype overlapping ATD and Joubert syndrome (213300), Lehman et al. (2010) found a shared homozygous 530-kb region on chromosome 15q13 that overlapped by about 310 kb with the region reported by Morgan et al. (2003). Sequencing of the coding regions of the MTMR10, MTMR15 (613534), and TRPM1 (603576) genes in 1 sib revealed no mutations. Sequencing of several other candidate genes in another patient with this phenotype also revealed no pathogenic mutations.