Neural Tube Defects, Folate-Sensitive
A number sign (#) is used with this entry because folate-sensitive neural tube defects (NTDFS) have been associated with variation in a number of genes involved in folate and homocysteine metabolism, including 5,10-methylenetetrahydrofolate reductase (MTHFR; 607093), methionine synthase (MTR; 156570), methionine synthase reductase (MTRR; 602568), and methylenetetrahydrofolate dehydrogenase-1 (MTHFD1; 172460).
See also 182940 for a discussion of neural tube defects that are not associated with folate metabolism. See also 601775 for a description of variation of folate levels in erythrocytes.
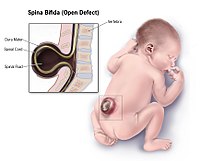
DescriptionNeural tube defects have a birth incidence of approximately 1 in 1,000 in American Caucasians and are the second most common type of birth defect after congenital heart defects. The most common NTDs are open spina bifida (myelomeningocele) and anencephaly (206500) (Detrait et al., 2005).
Women with elevated plasma homocysteine, low folate, or low vitamin B12 (cobalamin) are at increased risk of having a child with a neural tube defect (O'Leary et al., 2005). Motulsky (1996) cited evidence from the Centers for Disease Control ( Anonymous, 1992) that folic acid given before and during the first 4 weeks of pregnancy can prevent 50% or more of neural tube defects.
Botto et al. (1999) and Detrait et al. (2005) provided reviews of neural tube defects. De Marco et al. (2006) provided a detailed review of neurulation and the possible etiologies of neural tube defects.
PathogenesisMills et al. (1996) reviewed the possible biochemical mechanisms by which folic acid taken periconceptionally can prevent many neural tube defects. They cited evidence that NTDs do not result primarily from a nutritional deficiency of folic acid but rather from a metabolic defect or defects that can be corrected by a sufficiently large dose of folic acid. Their studies indicated that homocysteine metabolism is likely to be the critical pathway affected by folic acid. Mills et al. (1996) demonstrated significantly higher homocysteine levels in women carrying NTD-affected fetuses, suggesting that one of the enzymes involved in homocysteine metabolism is abnormal in NTD-affected pregnancies.
Molecular GeneticsVan der Put et al. (1995) found an increased frequency of the 677C-T thermolabile MTHFR polymorphism (607093.0003) among 55 Dutch patients with spina bifida and their parents compared to controls. Sixteen percent of mothers, 10% of fathers, and 13% of patients were homozygous for the variant compared to 5% of controls. Van der Put et al. (1995) concluded that the 677C-T variant is a genetic risk factor for spina bifida. Ou et al. (1996) studied fibroblast cultures from 41 NTD-affected fetuses and compared their genotypes with 109 blood specimens from the general population. They demonstrated that 677C-T homozygosity was associated with a 7.2-fold increased risk for NTD (p = 0.001). Ou et al. (1996) concluded that the 677C-T polymorphism of MTHFR may provide a partial biologic explanation for the prevention of neural tube defects by folic acid.
Christensen et al. (1999) assessed genotypes and folate status in 56 patients with spina bifida, 62 mothers of patients, 97 children without NTDs (controls), and 90 mothers of controls to determine the impact of these factors on NTD risk. In 20% of patients and 18% of mothers of patients, they found homozygosity for the MTHFR 677C-T polymorphism, compared to 11% of controls and 11% of control mothers, indicating that the mutant genotype conferred an increased risk for NTDs. The risk was further increased if both mother and child had this genotype. RBC folate was lower in patients and in mothers of patients compared to their respective controls. The combination of homozygous mutant MTHFR genotype and RBC folate in the lowest quartile conferred an odds ratio for being an NTD case of 13.43 and an odds ratio for having a child with NTD of 3.28. Christensen et al. (1999) proposed that the genetic-nutrient interaction, i.e., MTHFR polymorphism and low folate status, is associated with a greater risk for NTDs than either variable alone.
Among 56 patients with spina bifida and 58 mothers of children with spina bifida, Wilson et al. (1999) found that patients and mothers of patients were almost twice as likely to be homozygous for a 66A-G polymorphism in the MTRR gene (602568.0003) compared to controls. When combined with low levels of serum B12, the risk for mothers increased nearly 5 times (odds ratio of 4.8); the OR for children with this combination was 2.5. In the presence of combined MTHFR 677C-T and MTRR 66A-G homozygous mutant genotypes, children and mothers had a 4- and 3-fold increase in risk, respectively.
Doolin et al. (2002) analyzed data on the MTR 2756A-G polymorphism (156570.0008) and the MTRR 66A-G polymorphism and concluded that both variants influence the risk of spina bifida via the maternal rather than the embryonic genotype. For both variants, the risk of having a child with spina bifida appeared to increase with the number of high-risk alleles in the maternal genotype.
Relton et al. (2004) conducted a case-control association study in families affected by NTD to determine the contribution of polymorphic variation in genes involved in the folate-dependent homocysteine pathway. They investigated 7 polymorphisms in 6 genes: 2 in MTHFR and 1 each in MTRR, SHMT1 (182144), CBS (613381), GCP2 (600934), and RFC1 (600424). Both independent genetic effects and gene-gene interactions were observed in relation to NTD risk. Maternal-fetal interaction was also detected when offspring carried the MTHFR 677C-T variant and mothers carried the MTRR 66A-G variant.
Hol et al. (1998) identified a heterozygous mutation in the MTHFD1 gene (172460.0001) in 2 brothers with spina bifida, 1 with spina bifida aperta and 1 with spina bifida occulta. The unaffected maternal grandmother, mother, and a third brother also carried the mutation.
Brody et al. (2002) and De Marco et al. (2006) both observed an association between an R653Q polymorphism in the MTHFD1 gene (172460.0002) and neural tube defects in an Irish and Italian population, respectively. Parle-McDermott et al. (2006) analyzed the MTHFD1 gene in an independent sample of 245 Irish mothers with a history of NDT-affected pregnancy and 770 controls and found a significant excess of QQ homozygote mothers of NTD cases compared to controls (OR, 1.49; p = 0.019). Parle-McDermott et al. (2006) concluded that the R653Q polymorphism has a significant role in influencing a mother's risk of having an NTD-affected pregnancy in the Irish population.
O'Leary et al. (2005) found no association between the MTRR 66A-G polymorphism and neural tube defects in an Irish population comprising 470 patients and 447 mothers of patients. A dominant paternal effect was observed (OR, 1.46).
Mildly elevated maternal plasma homocysteine levels have been observed in some NTD pregnancies. In the NTD population in Ireland, Ramsbottom et al. (1997) examined the frequency of relatively common mutations in the gene encoding cystathionine beta-synthase (236200), one of the main enzymes that controls homocysteine levels. Neither the severely dysfunctional G307S CBS allele (236200.0001) nor the allele with the 68-bp insertion in exon 8 in association with the I278T CBS mutation (236200.0004) was observed in increased frequency in the cases relative to controls. Ramsbottom et al. (1997) concluded that loss-of-function CBS alleles do not account for NTD in Ireland.
Animal ModelTo investigate the mechanism whereby folate supplementation protects against heart and neural tube defects, Rosenquist et al. (1996) tested the effects of homocysteine on chick embryos and the effect of added folate. The hypothesis was that homocysteine may be the teratogenic agent, since serum homocysteine increases in folate depletion. Of embryos treated with homocysteine or homocysteine thiolactone, 27% showed neural tube defects. A high frequency of ventricular septal defects and neural tube defects was observed. Also, a ventral closure defect was found in a high percentage of day 9 embryos. The teratogenic dose was shown to raise serum homocysteine to over 150 nmol/ml, compared with a normal level of about 10 nmol/ml. Folate supplementation kept the rise in serum homocysteine to approximately 45 nmol/ml and prevented the teratogenic effect. Rosenquist et al. (1996) concluded that homocysteine per se causes dysmorphogenesis of the heart and neural tube, as well as of the ventral wall.
Carter et al. (1999) examined whether 'crooked tail' (Cd), a mouse strain prone to exencephaly, could provide a genetic animal model for folate-responsive neural tube defects. They localized the Cd locus to a 0.2-cM interval on mouse distal chromosome 6, identifying tightly linked markers for genotyping prior to phenotypic expression. In a controlled diet study, Cd was found to mimic closely the clinical response to folic acid observed in human populations. Folic acid supplementation reduced the recurrence risk of Cd exencephaly by as much as 55%. This rescue was dose dependent and did not require subjects to be inherently folate deficient. Like the female predominance of NTDs in humans, female Cd embryos were most likely to display exencephaly and were more responsive than males to the folic acid rescue. Importantly, folic acid supplementation shifted the severity of Cd phenotypic expression from early embryonic lethality to longer survival, and reduced the incidence of NTDs. Carter et al. (2005) found that the Cd mouse is caused by a heterozygous G494D mutation in a highly conserved region of the Lrp6 gene (603507). Functional expression studies showed that mutant Lrp6 protein resulted in Wnt (164820) hyperactivity. The findings provided a functional connection between Wnt signaling and folate rescue of neural tube defects in mouse, even though these proteins are not directly involved in folate metabolism.
Juriloff and Harris (2000) reviewed the numerous mouse models of NTDs, as well as the zonal pattern of neural tube closure and the effect of maternal nutrients on neural tube closure.