Xeroderma Pigmentosum, Complementation Group D
A number sign (#) is used with this entry because xeroderma pigmentosum complementation group D (XPD) is caused by homozygous or compound heterozygous mutation in the excision repair gene ERCC2 (126340) on chromosome 19q13.
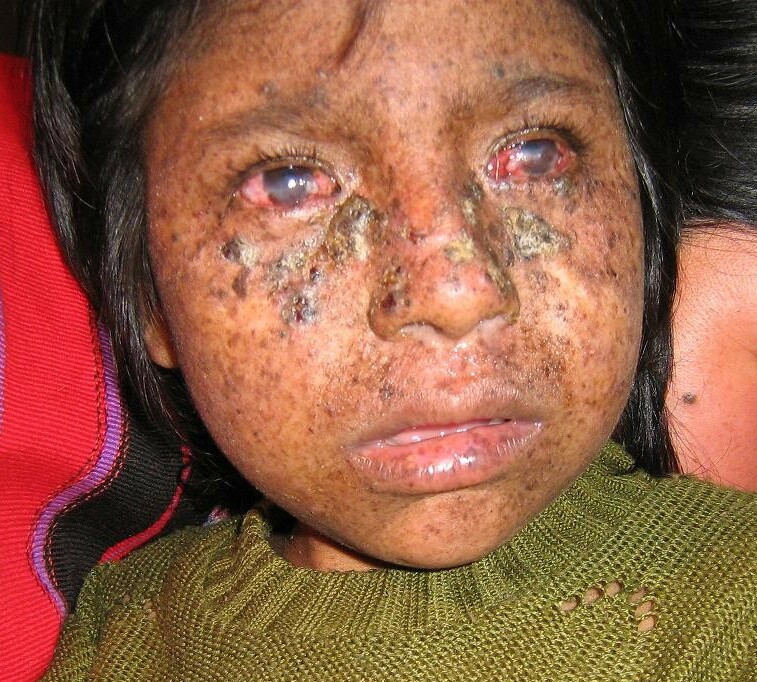
DescriptionXeroderma pigmentosum is a rare autosomal recessive disorder characterized by acute photosensitivity and a predisposition to skin cancer on sun-exposed areas of the body. The primary defect in XP involves nucleotide excision repair (NER) (summary by Flejter et al., 1992).
Clinical FeaturesIn a 31-year-old patient with XP classified as complementation group D by cell-fusion complementation methods, Ichihashi et al. (1988) described mild skin lesions and no apparent neurologic abnormalities despite the characteristic group D level of DNA repair deficiency.
Johnson and Squires (1992) stated that more than 30 unrelated individuals with XPD were known and that less than half of them showed major abnormalities of the central nervous system, once considered to be the hallmark of XPD.
Linkage of trichothiodystrophy (see TTD1, 601675) and XPD was suggested by Nuzzo et al. (1986) on the basis of 3 Italian families in which 4 individuals had both disorders. Study of surnames and genealogies suggested that the 3 sibships probably had a common ancestral couple.
Biochemical FeaturesStefanini et al. (1986) reported studies on the defect in DNA repair with creation of heterokaryons.
Arrand et al. (1989) cloned and characterized a hamster DNA repair gene that is able to confer an increase in resistance to ultraviolet (UV) irradiation on 2 XPD cell lines but not on an XPA (611153) line. They found no obvious similarities to 2 human excision repair genes, ERCC1 (126380) and ERCC2, which correct repair-defective hamster cells but have no effect on XP cells. Flejter et al. (1992) later found, however, that ERCC2 did correct the defect in XPD cells. Homologs of the hamster gene were identified in normal human genomic DNA and mRNA. The transcription pattern was not altered in XPD or XPA cells.
Molecular GeneticsSeetharam et al. (1987) used a shuttle vector plasmid to assess the types of mutations that cells from a patient with XPD would introduce into UV-damaged, replicating DNA. In comparison to UV-treated plasmids replicated in normal cells, there were fewer surviving plasmids, a higher frequency of plasmids with mutations, fewer plasmids with 2 or more mutations in the marker gene (coding for a tyrosine suppressor transfer RNA), and a new mutagenic hotspot. The major type of single-base mutation was G:C to A:T. Similar findings were reported with cells from a patient with xeroderma pigmentosum complementation group A (XPA; 278700).
In cell lines from patients with xeroderma pigmentosum group D, Frederick et al. (1994) identified mutations in the ERCC2 gene (126340.0001- 126340.0002).
In a Japanese patient with xeroderma pigmentosum group D, Kobayashi et al. (1997) identified compound heterozygosity for mutations in the ERCC2 gene (126340.0004-126340.0005).
Broughton et al. (2001) identified 2 patients with some features of both XP and TTD. A 3-year-old girl with sun sensitivity and mental and physical developmental delay was compound heterozygous for mutations in the ERCC2 gene (126340.0011-126340.0012). Cultured cells from this patient demonstrated barely detectable levels of nucleotide excision repair. The other patient, a 28-year-old woman with sun sensitivity, pigmentation changes, and skin cancers typical of XP, had an arg112-to-his mutation (R112H; 126340.0006) seen previously in TTD patients, and a leu485-to-pro mutation (L485P; 126340.0013) in the other allele. The level of repair of UV damage in the second patient was substantially higher than that in other patients with the same mutation. In both patients, polarized light microscopy revealed a tiger-tail appearance of the hair, and amino acid analysis of the hairshafts showed levels of sulfur-containing proteins between those of normal and TTD individuals.
HistoryMoshell et al. (1983) defined complementation group H xeroderma pigmentosum on the basis of a single patient who had both xeroderma pigmentosum and Cockayne syndrome. Johnson et al. (1989) found that hybrids between XPD cells and cells from groups A, B, C, E, F, G, and I showed cross-correction. However, no correction was observed when hybrids were created with XPH cells; thus, it is possible that XPD and XPH are allelic. Nonetheless, Robbins (1989) defended the separateness of XPH, quoting studies from the laboratory of Fujiwara indicating complete restoration of unscheduled DNA synthesis (UDS) in heterokaryons found between the group H strain and each of the 3 group D strains tested. Johnson (1989) continued to defend his negative result. Later, Robbins (1991) concluded that the so-called group H is indeed group D.