Neurofibromatosis 1
Summary
Clinical characteristics.
Neurofibromatosis 1 (NF1) is characterized by multiple café au lait spots, axillary and inguinal freckling, multiple cutaneous neurofibromas, iris Lisch nodules, and choroidal freckling. About half of people with NF1 have plexiform neurofibromas, but most are internal and not suspected clinically. Learning disabilities are present in at least 50% of individuals with NF1. Less common but potentially more serious manifestations include optic nerve and other central nervous system gliomas, malignant peripheral nerve sheath tumors, scoliosis, tibial dysplasia, and vasculopathy.
Diagnosis/testing.
The diagnosis of NF1 is usually based on clinical findings. Heterozygous pathogenic variants in NF1 are responsible for neurofibromatosis 1. Molecular genetic testing of NF1 is rarely needed for diagnosis.
Management.
Treatment of manifestations: Referral to specialists for treatment of complications involving the eye, central or peripheral nervous system, cardiovascular system, endocrine system, spine, or long bones; surgical removal of disfiguring or uncomfortable discrete cutaneous or subcutaneous neurofibromas. Surgical treatment of plexiform neurofibromas is often unsatisfactory. Complete surgical excision, when possible, of malignant peripheral nerve sheath tumors. Treatment of optic gliomas is generally unnecessary as they are usually asymptomatic and clinically stable. Dystrophic scoliosis often requires surgical management, whereas nondystrophic scoliosis can usually be treated conservatively. Methylphenidate treatment often benefits children with attention-deficit/hyperactivity disorder.
Surveillance: Annual physical examination by a physician familiar with the disorder; annual ophthalmologic examination in children, less frequently in adults; regular developmental assessment of children; regular blood pressure monitoring; MRI for follow up of clinically suspected intracranial tumors and other internal tumors. Begin annual mammography in women at age 30 with consideration of annual breast MRI in women between ages 30 and 50 years.
Genetic counseling.
NF1 is inherited in an autosomal dominant manner. Half of affected individuals have NF1 as the result of a de novo NF1 pathogenic variant. The offspring of an affected individual are at a 50% risk of inheriting the altered NF1 allele, but the disease manifestations are extremely variable, even within a family. Prenatal testing for a pregnancy at increased risk and preimplantation genetic testing are possible if the pathogenic variant in a family is known.
Diagnosis
Suggestive Findings
Neurofibromatosis 1 (NF1) should be suspected in individuals who have any of the following findings:
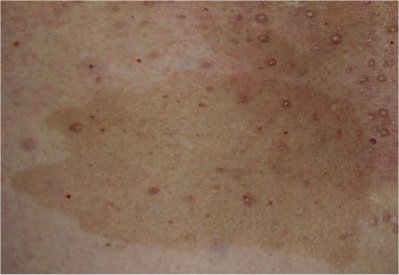
- Six or more café au lait macules (Figure 1) >5 mm in greatest diameter in prepubertal individuals and >15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas (Figure 2) of any type or one plexiform neurofibroma (Figure 3)
- Freckling in the axillary or inguinal regions
- Optic glioma
- Two or more Lisch nodules (iris hamartomas)
- A distinctive osseous lesion such as sphenoid dysplasia or tibial pseudarthrosis
- A first-degree relative (parent, sib, or offspring) with NF1 as defined by the above criteria

Figure 1.
Café au lait macules

Figure 2.
Neurofibromas

Figure 3.
Plexiform neurofibroma
Establishing the Diagnosis
The diagnosis of NF1 is established in a proband who meets the diagnostic criteria for neurofibromatosis 1 (NF1) developed by the National Institutes of Health [NIH 1988]. The NIH diagnostic criteria for NF1 are met in an individual who has two or more of the features listed in Suggestive Findings.
Note: As described below (Children), care must be taken in assigning the diagnosis of NF1 to a child with no known family history of NF1 since the diagnostic pigmentary findings overlap other disorders.
Adults. The NIH diagnostic criteria are both highly specific and highly sensitive in adults with NF1 [Ferner et al 2011, Ferner & Gutmann 2013].
Children
- Only about half of children with NF1 and no known family history of NF1 meet the NIH criteria for diagnosis by age one year; almost all do by age eight years [DeBella et al 2000a] because many features of NF1 increase in frequency with age.
- Children who have inherited NF1 from an affected parent can usually be identified within the first year of life because diagnosis requires just one feature in addition to a positive family history. This feature is usually multiple café au lait spots, which develop in infancy in more than 95% of individuals with NF1 [DeBella et al 2000b, Nunley et al 2009].
- Young children with multiple café au lait spots and no other NF1 features whose parents do not show signs of NF1 on careful physical and ophthalmologic examination should be strongly suspected of having NF1 and followed clinically as though they do [Nunley et al 2009].
- A definite diagnosis of NF1 can be made in most of these children by age four years using the NIH criteria.
- Young children who present with six or more café au lait macules and freckling in axillary or inguinal regions and who have no known family history of NF1 will meet the diagnostic criteria for NF1, but diagnoses of Legius syndrome or constitutional mismatch repair syndrome are also possible and need to be considered especially if no additional findings of NF1 develop with increasing age. See Differential Diagnosis.
Molecular genetic testing for identification of a heterozygous pathogenic variant in NF1 may be indicated in some individuals:
- Testing is indicated for individuals in whom NF1 is suspected but who do not fulfill the NIH diagnostic criteria. This is rarely necessary after early childhood.
- Testing may be useful in a young child with a serious tumor (e.g., optic glioma) in whom establishing a diagnosis of NF1 immediately would affect management.
- Testing of an adult with NF1 is necessary if prenatal or preimplantation genetic diagnosis in a current or future pregnancy is anticipated.
- In some families with spinal NF1 [Burkitt Wright et al 2013] or the NF1 c.2970-2972 delAAT pathogenic variant [Upadhyaya et al 2007, Quintáns et al 2011], affected individuals may not meet the NIH diagnostic criteria, especially in childhood. In such families, molecular testing is indicated for diagnosis of at-risk relatives.
Revision of the NIH diagnostic criteria has been recommended to take into account the availability of molecular testing for pathogenic variants of NF1 as well as clinical features (e.g., choroidal freckling, nevus anemicus, "unidentified bright objects") that often occur in childhood but were not known at the time of NIH Consensus Conference [Curless et al 1998, Ferrari et al 2014, Tadini et al 2014, Parrozzani et al 2015].
Single-gene testing. Sequence analysis of NF1 genomic DNA (gDNA) and/or cDNA (complementary DNA, copied from mRNA) is performed in association with gene-targeted deletion analysis.
- A multistep pathogenic variant detection protocol that combines analysis of genomic DNA and cDNA (mRNA) and testing for whole-gene or exon copy number changes is recommended if molecular genetic testing is indicated [Wimmer et al 2006, Messiaen & Wimmer 2008, Valero et al 2011, Sabbagh et al 2013]. This approach identifies more than 95% of NF1 pathogenic variants in individuals fulfilling the NIH diagnostic criteria. Because of the the variety and rarity of individual pathogenic variants found in people with NF1 and the frequency of pathogenic variants that affect splicing (22%-30%, more than 1/3 of which are not detected by gDNA sequencing) [Evans et al 2016], methods that include cDNA sequencing have higher detection rates than methods based solely on analysis of gDNA (Table 1).
- A multistep detection protocol that includes only cDNA (mRNA) sequencing and testing for whole-gene or exon copy number changes has been described that also has a diagnostic sensitivity of more than 95% [Evans et al 2016]. In this protocol, targeted gDNA sequencing is used to confirm that the pathogenic variants are in fact genomic.
- gDNA-only variant detection protocols are also available. These approaches, which involve sequencing of the entire NF1 coding region and adjacent splice sites from genomic DNA, have a somewhat lower diagnostic sensitivity, even if testing for whole-gene or exon copy number changes is also included [Maruoka et al 2014, van Minkelen et al 2014, Pasmant et al 2015, Zhang et al 2015, Calì et al 2017].
Chromosomal microarray analysis (CMA) may be performed to detect NF1 whole-gene deletions if the NF1 microdeletion phenotype is suspected clinically [Mautner et al 2010, Pasmant et al 2010, Kehrer-Sawatzki & Cooper 2012].
A multigene panel that includes NF1 and other genes of interest (see Differential Diagnosis) may also be considered. However, with the exception of SPRED1, the clinical phenotypes associated with constitutional variants of other genes on rasopathy, Noonan syndrome, or hereditary cancer panels are unlikely to overlap with that of NF1, and the detection frequency of pathogenic variants in NF1 on any panel performed by genomic DNA sequencing alone is likely to be stubstantially lower than that obtained by cDNA (mRNA) and targeted gDNA sequencing and copy number testing of NF1 with a multistep protocol. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview; thus, clinicians need to determine which multigene panel is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.
For an introduction to multigene panels click here. More detailed information for clinicians ordering genetic tests can be found here.
Note: Cytogenetic testing may be considered if a clinical diagnosis of NF1 is certain but no pathogenic variant is found on analysis of NF1 cDNA (mRNA), gDNA, and copy number. Cytogenetic rearrangements are responsible for NF1 in fewer than 1% of affected individuals, and many of the pathogenic changes that occur in these cases can probably be detected using multistep cDNA-based testing, as described above.
Table 1.
Molecular Genetic Testing Used in Neurofibromatosis 1
| Gene 1 | Method | Proportion of Probands with a Pathogenic Variant 2 Detectable by Method |
|---|---|---|
| NF1 | Multistep pathogenic variant detection protocol based on cDNA and gDNA sequence analysis 3, 4 | >95% 5 |
| Genomic DNA sequence analysis 3 | ~60%-90% 6 | |
| Gene-targeted deletion/duplication analysis 4 | ~5% 7 | |
| CMA | ~5% 7, 8 | |
| Cytogenetic analysis | <1% 9 |
- 1.
See Table A. Genes and Databases for chromosome locus and protein.
- 2.
See Molecular Genetics for information on allelic variants detected in this gene.
- 3.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 4.
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications.
- 5.
Messiaen & Wimmer [2008], Valero et al [2011], Sabbagh et al [2013], Evans et al [2016]
- 6.
Maruoka et al [2014], van Minkelen et al [2014], Pasmant et al [2015], Zhang et al [2015], Calì et al [2017]
- 7.
Whole-gene deletions occur in 4%-5% of individuals with NF1 [Kluwe et al 2004], and testing for whole NF1 deletions alone is sometimes performed when a "large-deletion phenotype" (see Genotype-Phenotype Correlations, "NF1 whole-gene deletion. . .") is suspected clinically [Mautner et al 2010, Pasmant et al 2010, Kehrer-Sawatzki & Cooper 2012].
- 8.
Detects large-scale (0.25- to 10-Mb) deletions or duplications. The pathogenic changes that occur in these cases can usually also be detected using multistep cDNA-based testing.
- 9.
Detects chromosome rearrangements that cannot usually be detected by CMA or multistep cDNA-based testing. Most NF1 whole-gene deletions cannot be detected by cytogenetic analysis.
Clinical Characteristics
Clinical Description
The clinical manifestations of neurofibromatosis 1 (NF1) are extremely variable [Ferner et al 2011, Ferner & Gutmann 2013, Dunning-Davies & Parker 2016].
Cutaneous Features
Café au lait spots and freckling. Multiple café au lait spots occur in nearly all affected individuals, and intertriginous freckling develops in almost 90%.
Typically, the characteristic café au lait spots in individuals with NF1 are ovoid in shape with well-defined borders, uniform in color (a little darker than the background pigmentation of the individual's skin), and about 1-3 cm in size; however, they may be smaller or much larger, lighter or darker, or irregular in shape. The pigmentation may also be irregular, with freckling or a more deeply pigmented smaller café au lait spot within a larger more typically colored lesion. Café au lait spots are flat and flush with the surrounding skin; if the skin of the lesion is raised or has an unsually soft or irregular texture in comparison to the surrounding skin, an underlying plexiform neurofibroma is likely. The darker pigmentation of café au lait spots may be difficult to see in people with very fair skin or very dark skin, where the color of the lesions is similar to that of the rest of the skin. A Wood's light is useful in such cases to demonstrate the pigmented macules. Café au lait spots are not seen on the palms or soles in people with NF1 but can occur almost anywhere else on the body.
Clusters of freckles are frequent in sun-exposed areas and may also be seen diffusely over the trunk, proximal extremities, and neck in people with NF1. Similar freckling is common in fair-skinned people who do not have NF1. However, people with NF1 also develop freckles in areas where skin rubs against skin – in the axilla, groin, and under the breasts in women. These freckles look like any others: it is only their location that is unusual.
Neurofibromas. Numerous benign cutaneous neurofibromas are usually present in adults with NF1.
Discrete cutaneous and subcutaneous neurofibromas are rare before late childhood. The total number of neurofibromas seen in adults with NF1 varies from a few to hundreds or even thousands. Additional cutaneous and subcutaneous neurofibromas continue to develop throughout life, although the rate of appearance may vary greatly from year to year. Many women experience a rapid increase in the number and size of neurofibromas during pregnancy [Roth et al 2008].
About half of people with NF1 have plexiform neurofibromas, but most are internal and not suspected clinically [Tonsgard et al 1998, Mautner et al 2008, Plotkin et al 2012]. Most of these tumors grow slowly if at all over periods of years, but very rapid growth can occur in benign lesions, especially in early childhood [Dombi et al 2007, Tucker et al 2009a, Nguyen et al 2012]. When symptomatic, plexiform neurofibromas can cause disfigurement and may compromise function or even jeopardize life.
Other skin findings. Juvenile xanthogranuloma and nevus anemicus are more common than expected in people with NF1 and may be useful in supporting the diagnosis in young children who do not meet the standard diagnostic criteria [Marque et al 2013, Ferrari et al 2014, Hernández-Martín et al 2015, Vaassen & Rosenbaum 2016]. Juvenile xanthogranulomas are small, tan- or orange-colored papules that may occur in clusters. Nevus anemicus is an irregularly shaped macule that is paler than surrounding skin and that does not get red when rubbed, as the skin surrounding it does.
Ocular Findings
Ocular manifestations of NF1 include optic gliomas, which may lead to blindness, Lisch nodules, and choroidal freckling. Lisch nodules are innocuous iris hamartomas that can be demonstrated on slit lamp examination in almost all adults but in fewer than half of children with NF1 younger than age five years [Ragge et al 1993]. Choroidal freckling cannot be seen on standard opthalmologic examination but can be visualized by scanning laser ophthalmoscopy with infrared or near-infrared light, infrared reflectance imaging, or optical coherence tomography [Vagge et al 2016]. The lesions, which are Schwann cell proliferations arrayed in concentric rings around an axon, occur in the majority of people with NF1 of all ages and increase in prevalence and extent with age. Infrequent ocular manifestations of NF1 include retinal vasoproliferative tumors [Hood et al 2009, Shields et al 2014] and neovascular glaucoma [Elgi et al 2010, Chiu et al 2011, Al Freihi et al 2013].
Symptomatic optic pathway gliomas in individuals with NF1 usually present before age six years with loss of visual acuity, proptosis, or strabismus, but these tumors may not become symptomatic until later in childhood or even in adulthood [Friedrich & Nuding 2016]. Symptomatic optic pathway gliomas in NF1 are frequently stable for many years or only very slowly progressive; some of these tumors even spontaneously regress [Listernick et al 2007, Shamji & Benoit 2007, Nicolin et al 2009, Sellmer et al 2018].
Neurologic Manifestations
For a discussion of peripheral nerve and central nervous system tumors see Cutaneous Features and Tumors.
Most individuals with NF1 have normal intelligence, but learning disabilities or behavioral problems occur in 50%-80% [Pride & North 2012, Lehtonen et al 2013]. Frank intellectual disability is seen in 6%-7%, a frequency about twice that in the general population [Pride & North 2012, Lehtonen et al 2013]. Features of autism spectrum disorder occur in up to 30% of children with NF1 [Garg et al 2013a, Garg et al 2013b, Walsh et al 2013, Plasschaert et al 2015, Morris et al 2016]. A variety of other learning and behavioral problems that persist into adulthood have been described [Descheemaeker et al 2013, Pride et al 2013, Granström et al 2014]. Deficits in visual-spatial performance, social competence, and attention are most commonly seen in people with NF1, but problems with motor function, executive function, memory, and language are also frequent [Pride & North 2012, Lehtonen et al 2013].
Some people with NF1 develop a diffuse polyneuropathy, often in association with multiple nerve root tumors [Drouet et al 2004, Ferner et al 2004]. Affected patients are at high risk for malignant peripheral nerve sheath tumors.
Seizures are more common in people with NF1 than in the general population and can occur at any age [Hsieh et al 2011, Ostendorf et al 2013]. The seizures are usually focal and may be associated with the presence of a brain tumor or area of infarction [Ostendorf et al 2013]. Control of focal seizures in people with NF1 may require the use of more than one antiepileptic drug or surgical removal of the affected part of the brain [Ostendorf et al 2013, Gales & Prayson 2017].
Sleep disturbance is frequent in people with NF1 [Leschziner et al 2013, Licis et al 2013, Maraña Pérez et al 2015]. Headaches, including migraine headaches, are also very common [Pinho et al 2014, Afridi et al 2015]. Pain in association with plexiform neurofibromas is also common [Kim et al 2009, Tucker et al 2009a] and must be distinguished from the pain that may be the first sign of transformation to a malignant peripheral nerve sheath tumor.
Musculoskeletal Features
Generalized osteopenia is more common than expected in people with NF1, and osteoporosis appears to be both more common and earlier in onset than in the general population [Tucker et al 2009b, Heervä et al 2012, Petramala et al 2012, Armstrong et al 2013, Heervä et al 2013]. The pathogenesis of these bony changes is not fully understood, but individuals with NF1 have often been found to have lower-than-expected serum 25-hydroxyvitamin D concentrations, elevated serum parathyroid hormone levels, and evidence of increased bone resorption [Lammert et al 2006, Brunetti-Pierri et al 2008, Stevenson et al 2008, Tucker et al 2009b, Stevenson et al 2011, Heervä et al 2012, Petramala et al 2012]. The function of both osteoblasts and osteoclasts appears to be abnormal in bone from people with NF1 [Seitz et al 2010, Kühnisch et al 2014].
Dysplasia of the long bones, most often the tibia and fibula, is an infrequent but characteristic feature of NF1 [Elefteriou et al 2009]. The lesion is congenital and is almost always unilateral. It usually presents in infancy with anteriolateral bowing of the lower leg, which is quite different from the common physiologic bowing seen in children when they begin to walk. Early recognition of tibial dysplasia permits bracing, which may prevent fracture. The initial radiographic changes are narrowing of the medullary canal with cortical thickening at the apex of the bowing [Stevenson et al 2007]. Long-bone dysplasia appears to reflect an abnormality of the bone itself and is not usually associated with adjacent neurofibromas. In contrast, sphenoid wing dysplasia and vertebral dysplasia – the other two characteristic focal bony lesions of NF1 – are associated with adjacent plexiform neurofibroma or dural ectasia (or both) [Alwan et al 2005, Arrington et al 2013, Nguyen et al 2015, Hu et al 2016].
Sphenoid wing dysplasia may be detected incidentally on cranial imaging or present as strabismus or asymmetry of the orbits. It is often static but may be progressive, occasionally disrupting the integrity of the orbit and producing pulsating enophthalmos [Friedrich et al 2010].
Scoliosis in NF1 may be of either the dystrophic or nondystrophic type [Elefteriou et al 2009]. The latter resembles common adolescent scoliosis and is not associated with vertebral anomalies. Dystrophic scoliosis occurs at a much younger age (typically age 6-8 years), is characterized by an acute angle over a short segment of the spine, and may be very rapidly progressive.
Healing of fractured or defective bone in any of these focal lesions is often unsatisfactory; treatment is frequently difficult [Pessis et al 2015, Borzunov et al 2016], and best accomplished by experienced specialists.
Children with NF1 have reduced muscle strength when compared to unaffected children of the same age, sex, and weight [Summers et al 2015].
Vascular Involvement
Hypertension is common in NF1 and may develop at any age [Friedman et al 2002, Lama et al 2004]. In most cases, the hypertension is "essential," but a characteristic NF1 vasculopathy can produce renal artery stenosis, coarctation of the aorta, or other vascular lesions associated with hypertension. A renovascular cause is often found in children with NF1 and hypertension [Fossali et al 2000, Han & Criado 2005].
Stroke is more common and often occurs at a younger age among people with NF1 than in the general population [Terry et al 2016]. NF1 vasculopathy involving major arteries or arteries of the heart or brain can have serious or even fatal consequences [Cairns & North 2008, Rea et al 2009, Stansfield et al 2012, Koss et al 2013].
- Anatomically variant stenotic or ectatic cerebral arteries and intracranial aneurysms occur more frequently in individuals with NF1 than in the general population [Rosser et al 2005, Schievink et al 2005, Bekiesińska-Figatowska et al 2014, D'Arco et al 2014].
- Cerebrovascular abnormalities in NF1 typically present as stenoses or occlusions of the internal carotid, middle cerebral, or anterior cerebral artery.
- Small telangiectatic vessels form around the stenotic area and appear as a "puff of smoke" (moya-moya) on cerebral angiography.
- Moya-moya develops about three times more often than expected in children with NF1 after cranial irradiation for primary brain tumor [Ullrich et al 2007b, Murphy et al 2015].
Cardiac Issues
Valvar pulmonic stenosis is more common in individuals with NF1 than in the general population [Lin et al 2000]. Congenital heart defects or hypertrophic cardiomyopathy may be especially frequent among persons with NF1 whole-gene deletions [Nguyen et al 2013b]. Adults with NF1 may develop pulmonary hypertension, often in association with parenchymal lung disease, another late-onset but potentially quite serious feature of NF1 [Stewart et al 2007, Zamora et al 2007, Montani et al 2011, Ennibi et al 2015]. Intracardiac neurofibromas may also occur [Nguyen et al 2013b].
Tumors
Neurofibromas are benign Schwann cell tumors that can affect virtually any nerve in the body [Stemmer-Rachamimov & Nielsen 2012]. Cutaneous neurofibromas develop in almost all people with NF1 and increase in number ‒ and very slowly in size ‒ with age. About half of people with NF1 have plexiform neurofibromas, but in most cases they are internal, and thus not apparent on clinical examination. The extent of plexiform neurofibromas seen on the surface of the body often cannot be determined by clinical examination alone. MRI is the method of choice for imaging plexiform neurofibromas (see Imaging).
Plexiform neurofibromas tend to grow in childhood and adolescence and then remain stable throughout adulthood [Dombi et al 2007, Tucker et al 2009a, Nguyen et al 2012]. Although most plexiform neurofibromas are asymptomatic, they may cause pain, grow to enormous size, cause serious disfigurement, produce overgrowth or erosion of adjacent tissue, or impinge on the function of nerves and other structures.
Malignant peripheral nerve sheath tumors are the most frequent malignant neoplasms associated with NF1, occurring in approximately 10% of affected individuals [Rasmussen et al 2001, Evans et al 2002, Walker et al 2006, Friedrich et al 2007, McCaughan et al 2007]. In comparison to the general population, malignant peripheral nerve sheath tumors tend to occur at a younger age in people with NF1, often in adolescence or early adulthood [Hagel et al 2007, McCaughan et al 2007, Valentin et al 2016]. Individuals with NF1 who have a whole-gene deletion [De Raedt et al 2003, Kluwe et al 2003, Kehrer-Sawatzki et al 2012], who have benign subcutaneous neurofibromas, or whose burden of benign internal plexiform neurofibromas is high appear to be at greater risk of developing malignant peripheral nerve sheath tumors than people with NF1 who do not have these features [Tucker et al 2005, Mautner et al 2008, Plotkin et al 2012, Nguyen et al 2014].
The most common neoplasms apart from benign neurofibromas in people with NF1 are optic nerve gliomas and brain tumors [Prada et al 2015, Blanchard et al 2016, Friedrich & Nuding 2016, Parkhurst & Abboy 2016, Sellmer et al 2017, Sellmer et al 2018]. Optic gliomas in people with NF1 are usually asymptomatic and remain so throughout life. In fact, the majority of these lesions appear to regress spontaneously – their prevalence declines from approximately 20% in young children to less than 5% in older adults with NF1 [Sellmer et al 2018]. The clinical course in patients with optic giomas tends to be milder in patients with NF1 than in those who do not have have NF1 [Mandiwanza et al 2014]. Second central nervous system gliomas occur in 17%-20% of individuals with NF1 who have optic pathway gliomas [Sharif et al 2006, Sellmer et al 2018].
Brain stem and cerebellar gliomas in individuals with NF1 may also follow a less aggressive course than in those who do not have NF1 [Ullrich et al 2007a, Sellmer et al 2017]. About 20% of people with NF1 who have one non-optic glioma have two or more of these tumors [Sellmer et al 2017]. Non-optic gliomas and malignant peripheral nerve sheath tumors within the field of treatment are substantially more common in NF1 patients with gliomas who are treated with radiotherapy [Kleinerman 2009, Madden et al 2014]. Transformation of a pilocytic astrocytoma to a more malignant brain tumor may also occur after radiatiotherapy in patients with NF1 [Krishnatry et al 2016].
Leukemia (especially juvenile chronic myelogenous leukemia) and myelodysplastic syndromes are infrequent in children with NF1 but much more common than in children without NF1. A variety of other tumors may also be seen more often than expected in individuals with NF1, including rhabdomyosarcomas [Crucis et al 2015], pheochromocytomas [Gorgel et al 2014], gastrointestinal stromal tumors [Andersson et al 2005, Takazawa et al 2005, Miettinen et al 2006, Gorgel et al 2014, Nishida et al 2016], glomus tumors [Harrison et al 2013, Kumar et al 2014], and retinal vasoproliferative tumors [Shields et al 2014]. Women with NF1 have a substantially increased risk of developing breast cancer before age 50 years [Madanikia et al 2012, Wang et al 2012, Seminog & Goldacre 2015] and of dying of breast cancer [Evans et al 2011]. People with NF1 may also be at increased risk for other cancers [Seminog & Goldacre 2013, Varan et al 2016].
Age of Onset of Manifestations
Many individuals with NF1 develop only cutaneous manifestations of the disease and Lisch nodules, but the frequency of more serious complications increases with age. Various manifestations of NF1 have different characteristic times of appearance [DeBella et al 2000b, Boulanger & Larbrisseau 2005, Williams et al 2009, Ferner et al 2011]. For example:
- Bony manifestations such as anteriolateral tibial bowing are congenital.
- Café au lait spots are often present at birth and increase in number during the first few years of life.
- Diffuse plexiform neurofibromas of the face and neck rarely appear after age one year, and diffuse plexiform neurofibromas of other parts of the body rarely develop after adolescence.
- Deep nodular plexiform neurofibromas may be seen at any age but are usually not symptomatic in childhood and often remain asymptomatic in adulthood.
- Optic gliomas develop in the first six years of life.
- The rapidly progressive (dysplastic) form of scoliosis almost always develops between ages six and ten years, although milder forms of scoliosis without vertebral anomalies typically occur during adolescence.
- Malignant peripheral nerve sheath tumors usually occur in adolescence or adulthood.
Growth
Individuals with NF1 tend to be below average in height and above average in head circumference for age [Clementi et al 1999, Szudek et al 2000a, Szudek et al 2000b, Virdis et al 2003, Karvonen et al 2013, Soucy et al 2013]. However, few individuals with NF1 have height more than 3 SD below the mean or head circumference more than 4 SD above the mean. People whose NF1 is caused by a deletion of the entire NF1 locus show a different pattern, with overgrowth (especially in height) between ages two and six years [Mautner et al 2010, Pasmant et al 2010, Kehrer-Sawatzki & Cooper 2012, Ning et al 2016]. The clinical features in some of these individuals resemble those of Weaver syndrome.
Pubertal development is usually normal, but precocious puberty may occur in children with NF1, especially in those with tumors of the optic chiasm [Virdis et al 2000, Kocova et al 2015]. Delayed puberty is also common [Virdis et al 2003].
Life Expectancy
The median life expectancy of individuals with NF1 is approximately eight years lower than in the general population [Evans et al 2011, Wilding et al 2012]. Malignancy (especially malignant peripheral nerve sheath tumors) and vasculopathy are the most important causes of early death in individuals with NF1 [Zöller et al 1995, Rasmussen et al 2001, Evans et al 2011, Masocco et al 2011].
Quality of Life
Quality of life assessments are lower in both children and adults with NF1 than in comparison groups [Vranceanu et al 2013, Merker et al 2014, Vranceanu et al 2015]. Cosmetic, medical, social, and behavioral features of the disease all may compromise the quality of life in people with NF1, and clinical depression may impair their ability to function effectively [Cohen et al 2015].
Imaging
Note: The value of performing routine head MRI scanning in individuals with NF1 at the time of diagnosis is controversial.
- Proponents state that such studies are useful in helping to establish the diagnosis in some individuals, in identifying any structural anomaly of the brain or skull, tumors, or vascular disease before it becomes clinically apparent in others, and in evaluating the context in which extracranial complications occur in still others.
- Those who oppose routine head MRI scanning point to the uncertain clinical significance of features such as UBOs ("unidentified bright objects"), the cost of such imaging, and the requirement for sedation in small children. Although clinical management should not be affected by the presence of intracranial lesions such as UBOs or optic nerve thickening in asymptomatic individuals with NF1, finding such lesions may result in regularly repeating the MRI for reassurance despite the continued absence of related symptoms, adding further to the cost as well as to the anxiety of the individual and family, without any benefit.
MRI is the method of choice for demonstrating the size and extent of plexiform neurofibromas [Mautner et al 2008, Cai et al 2009, Matsumine et al 2009, Van Meerbeeck et al 2009, Plotkin et al 2012, Hirbe & Gutmann 2014] and for monitoring their growth over time [Dombi et al 2007, Tucker et al 2009a, Nguyen et al 2012]. MRI is also useful in characterizing optic pathway gliomas, other brain tumors, structural abnormalities of the brain, and signs of cerebrovascular disease in people with NF1 [Cairns & North 2008, Rea et al 2009, Lin et al 2011, Prada et al 2015, Blanchard et al 2016, Sellmer et al 2017, Sellmer et al 2018]. MR angiography is valuable in assessing NF1 vasculopathy [D'Arco et al 2014]. Conventional radiographic studies can demonstrate the skeletal anomalies that occur in people with NF1 [Patel & Stacy 2012], but CT imaging or three-dimensional CT reconstructions may be necessary when surgical treatment of bony lesions is being planned. PET and CT/PET can help to distinguish benign and malignant peripheral nerve sheath tumors [Combemale et al 2014, Hirbe & Gutmann 2014, Salamon et al 2014, Chirindel et al 2015, Salamon et al 2015, Van Der Gucht et al 2016], but definitive differentiation can only be made by histologic examination of the tumor. CT/PET appears to be useful in guiding percutaneous biopsies of peripheral nerve sheath tumors suspected of being malignant [Brahmi et al 2015].
MRI studies have shown that people with NF1 have larger brains, on average, than people without NF1, but in NF1 gray matter volume is not correlated with IQ [Greenwood et al 2005, Margariti et al 2007, Karlsgodt et al 2012]. Enlargement of the corpus callosum is seen in some children with NF1 and has been associated with learning disabilities [Pride et al 2010, Aydin et al 2016]. More tortuosity of the optic nerve is seen on MRI in children with NF1 than in those without NF1, but optic nerve tortuosity is not associated with the occurrence of optic glioma among patients with NF1 [Ji et al 2013]. Diffusion tensor imaging has shown abnormalities of white matter microstructure, especially in the frontal lobes and corpus callosum [Ferraz-Filho et al 2012b, Karlsgodt et al 2012, Nicita et al 2014, Aydin et al 2016], and functional MRI studies have demonstrated altered connectivity in people with NF1 [Tomson et al 2015]. Individuals with NF1 also exhibit metabolic alterations in comparison to controls on magnetic resonance spectroscopy (MRS) [Nicita et al 2014, Rodrigues et al 2015].
The clinical significance of the so-called "unidentified bright objects" (UBOs) visualized on brain MRI in more than 50% of children with NF1 is uncertain [Sabol et al 2011, Friedrich & Nuding 2016, Sellmer et al 2018]. These hyperintense lesions seen on T2-weighted imaging may occur in the optic tracts, basal ganglia, brain stem, cerebellum, or cortex, and usually show no evidence of a mass effect. Typical UBOs are not seen on T1-weighted MRI imaging or on CT scan. UBOs show signs of intramyelinic edema on diffusion-weighted MRI [Ferraz-Filho et al 2012a, Ferraz-Filho et al 2012b, Billiet et al 2014, Ertan et al 2014] and MRS [Rodrigues et al 2015] and correspond pathologically to areas of spongiform myelinopathy [DiPaolo et al 1995]. They may disappear with age and are less common in adults than in children with NF1 [Payne et al 2014, Friedrich & Nuding 2016, Sellmer et al 2018].
The presence of UBOs does not appear to be related to the occurrence of seizures in children with NF1 [Hsieh et al 2011]. Some studies have suggested that the presence, number, volume, location, or disappearance of UBOs over time correlates with learning disabilities in children with NF1, but findings have not been consistent across investigations [Hyman et al 2007, Chabernaud et al 2009, Feldmann et al 2010, Payne et al 2014, Roy et al 2015].
Genotype-Phenotype Correlations
NF1 is characterized by extreme clinical variability, not only between unrelated individuals and among affected individuals within a single family but even within a single person with NF1 at different times in life. Only a few clear correlations have been observed between particular pathogenic NF1 alleles and consistent clinical phenotypes [Shofty et al 2015]:
- NF1 whole-gene deletion is associated with large numbers and early appearance of cutaneous neurofibromas, more frequent and more severe cognitive abnormalities, somatic overgrowth, large hands and feet, and dysmorphic facial features [Mautner et al 2010, Pasmant et al 2010, Kehrer-Sawatzki & Cooper 2012].
- A 3-bp in-frame deletion of exon 17 (c.2970-2972 delAAT) (NF Consortium nomenclature; exon 22 of NCBI nomenclature) is associated with typical pigmentary features of NF1 but no cutaneous or surface plexiform neurofibromas [Upadhyaya et al 2007].
- Any one of several missense variants affecting NF1 codon Arg1809 (see Table 2) in exon 29 (NF Consortium nomenclature; exon 38 of NCBI nomenclature) is associated with multiple café au lait spots, learning disabilities, short stature, and pulmonic stenosis but absence of cutaneous neurofibromas or clinically apparent plexiform neurofibromas [Pinna et al 2015, Rojnueangnit et al 2015].
Persons with NF1 (including those with NF1/Noonan syndrome or Watson syndrome phenotypes) who also have pulmonic stenosis appear to have nontruncating NF1 variants more frequently than the truncating variants that are found more often in other persons with NF1 [Ben-Shachar et al 2013].
The consistent familial transmission of NF1 variants such as Watson syndrome (multiple café au lait spots, pulmonic stenosis, and intellectual disability) [Allanson et al 1991, Tassabehji et al 1993] and familial spinal neurofibromatosis [Upadhyaya et al 2009, Burkitt Wright et al 2013, Ruggieri et al 2015] also indicates that allelic heterogeneity plays a role in the clinical variability of NF1. Statistical analysis of the NF1 phenotype within and between families [Sabbagh et al 2009, Sabbagh et al 2013] and observations on 23 half-sibs fathered by a sperm donor with mosaic NF1 [Ejerskov et al 2016] suggest that the NF1 pathogenic allele itself accounts for only a small fraction of phenotypic variation. Differences in expression of the normal NF1 allele may account for some of the phenotypic variability [Jentarra et al 2012]. Statistical analysis of clinical features in affected families [Pasmant et al 2012] and studies of polymorphisms in putative epistatic loci [Pemov et al 2014] suggest that modifying genes at other loci influence many aspects of the NF1 phenotype.
The extreme clinical variability of NF1 suggests that random events are important in determining the phenotype of affected individuals. Evidence in support of this interpretation is provided by the occurrence of acquired "second hit" variants or loss of heterozygosity at the NF1 locus in some neurofibromas, malignant peripheral nerve sheath tumors, pheochromocytomas, astrocytomas, gastrointestinal stromal tumors, myeloid malignancies, mandibular giant cell granulomas, and glomus tumors from patients with NF1 [Upadhyaya et al 2012, Emmerich et al 2015]. NF1 loss of heterozygosity has also been observed in some instances in melanocytes grown from café au lait spots [Maertens et al 2007, De Schepper et al 2008], macronodular adrenal hyperplasia, and tissue associated with tibial pseudarthrosis [Kobus et al 2015] from patients with NF1 [Lee et al 2012, Paria et al 2014, Sant et al 2015].
It seems likely that the clinical variability of NF1 results from a combination of genetic, non-genetic, and stochastic factors. Such complexity and the diversity of constitutional NF1 pathogenic variants that occur in this disease will continue to make genotype-phenotype correlation difficult.
Penetrance
Penetrance is virtually complete after childhood.
Nomenclature
NF1 was previously referred to as peripheral neurofibromatosis, to distinguish it from NF2 (central neurofibromatosis) – although central nervous system involvement may also occur in NF1.
"Neurofibromatosis" without further specification is sometimes used in the literature to refer to NF1, but this usage is confusing because other authors employ the term "neurofibromatosis" to designate a group of conditions that includes (in addition to NF1) NF2, schwannomatosis, and other clinically similar disorders.
Prevalence
NF1 is one of the most common dominantly inherited genetic disorders, occurring with an incidence at birth of approximately one in 3000 individuals [Lammert et al 2005, Evans et al 2010].
Almost half of all affected individuals have the disorder as the result of de novo mutation. The mutation rate for NF1 (~1:10,000) is among the highest known for any gene in humans. The cause of the unusually high mutation rate is unknown.
Differential Diagnosis
More than 100 genetic conditions and multiple congenital anomaly syndromes that include café au lait spots or other individual features of neurofibromatosis 1 (NF1) have been described, but few of these disorders are ever confused with NF1.
Conditions most frequently confused with NF1
- Legius syndrome, an autosomal dominantly inherited condition that includes multiple café au lait spots, axillary freckling, macrocephaly, and, in some individuals, facial features that resemble Noonan syndrome [Brems et al 2012] caused by heterozygous pathogenic variants in SPRED1. Affected individuals may meet the diagnostic criteria for NF1, but Lisch nodules, neurofibromas, and central nervous system tumors do not usually occur. About 8% of children with six or more café au lait spots and no other clinical features of NF1 have Legius syndrome [Evans et al 2016]. Distinguishing Legius syndrome from NF1 is sometimes impossible on the basis of clinical features alone in a young child because the multiple cutaneous neurofibromas and Lisch nodules that characterize most patients with NF1 do not usually arise until later in childhood or adolescence. Examination of the parents for signs of Legius syndrome or NF1 may distinguish the two conditions, but in sporadic cases reevaluation of the patient after adolescence or molecular testing may be necessary to establish the diagnosis.
- Constitutional mismatch repair deficiency (OMIM 276300) associated with homozygosity or compound heterozygosity for a pathogenic variant in one of the genes causing Lynch syndrome [Wimmer et al 2017]. The cutaneous phenotype is remarkably similar to NF1, and affected individuals may meet the NIH diagnostic criteria for NF1. However, individuals homozygous for pathogenic variants associated with Lynch syndrome usually develop tumors that are typical of Lynch syndrome but with a younger age of onset than seen in Lynch syndrome heterozygotes. This condition is distinguishable from NF1 in that the parents are often consanguineous and one or both parents often have clinical findings and/or a family history of Lynch syndrome. Typically, neither parent has clinical findings of NF1. A pathogenic variant in NF1 is usually not demonstrable in the blood of these patients.
- Piebald trait (OMIM 172800), characterized by areas of cutaneous pigmentation and depigmentation with hyperpigmented borders of the unpigmented areas, and white forelock. Some individuals with this autosomal dominant condition meet the diagnostic criteria for NF1 [Stevens et al 2012].
- Neurofibromatosis 2 (NF2), characterized by bilateral vestibular schwannomas, schwannomas of other cranial and peripheral nerves, cutaneous schwannomas, meningiomas, and juvenile posterior subcapsular cataract. NF2 is genetically and clinically distinct from NF1. The disorder is caused by pathogenic variants in NF2 and inherited in an autosomal dominant manner.
- Schwannomatosis (multiple schwannomas of cranial, spinal or peripheral nerves, usually without vestibular, ocular or cutaneous features of NF2) [Merker et al 2012]
- Multiple café au lait spots (an autosomal dominant trait without other features of neurofibromatosis) (OMIM 114030). The families described have not been tested for SPRED1 pathogenic variants; thus it is not known if this condition is distinct from Legius syndrome, which has a similar phenotype.
- Noonan syndrome with multiple lentigines (NSML), an autosomal dominant disorder previously referred to as LEOPARD syndrome and characterized by multiple lentigines, ocular hypertelorism, deafness, and congenital heart disease. NSML is caused by a pathogenic variant in one of four genes (BRAF, MAP2K1, PTPN11, and RAF1).
- Fibrous dysplasia/McCune-Albright syndrome (FD/MAS), characterized by large café au lait spots with irregular margins and polyostotic fibrous dysplasia. FD/MAS is the result of early embryonic postzygotic somatic activating mutation of GNAS. There are no verified instances of vertical transmission of FD/MAS.
- Noonan syndrome, an autosomal dominant disorder characterized by short stature, congenital heart defect, neck webbing, and characteristic facies. Noonan syndrome is caused by pathogenic variants in BRAF, KRAS, MAP2K1, NRAS, PTPN11, RAF1, RIT1, or SOS1. (A Noonan syndrome phenotype also occurs in ~12% of individuals with NF1 [Colley et al 1996]; see Genetically Related Disorders.)
- Infantile myofibromatosis (OMIM PS228550), an autosomal dominant condition characterized by multiple tumors of the skin, subcutaneous tissues, skeletal muscle, bones, and viscera.
- Proteus syndrome, characterized by hamartomatous overgrowth of multiple tissues, connective tissue nevi, epidermal nevi, and hyperostoses. A somatic mosaic AKT1 pathogenic variant has been identified in more than 90% of individuals meeting Proteus syndrome diagnostic criteria.
- Multiple orbital neurofibromas, painful peripheral nerve tumors, distinctive face, and marfanoid habitus [Babovic-Vuksanovic et al 2012]
Management
Evaluations Following Initial Diagnosis
To establish the extent of disease and needs in an individual diagnosed with neurofibromatosis 1 (NF1), the following evaluations are recommended:
- Personal medical history with particular attention to features of NF1
- Physical examination with particular attention to the skin, skeleton, cardiovascular system, and neurologic systems
- Ophthalmologic evaluation including slit lamp examination of the irides and infrared reflectance imaging or optical coherence tomography of the fundus
- Developmental assessment in children
- Other studies as indicated on the basis of clinically apparent signs or symptoms
- Consultation with a clinical geneticist and/or genetic counselor
In addition, a family history with particular attention to features of NF1 should be obtained, and physical examinations and ophthalmologic examinations (including slit lamp exams and infrared reflectance imaging or optical coherence tomography of the fundus) should be performed on both parents to determine if the condition in the affected individual was inherited or occurred de novo. This determination is necessary for genetic counseling and may help identify patients who have a condition that is not caused by pathogenic variants in NF1, such as Legius syndrome or constitutive mismatch repair deficiency (see Differential Diagnosis).
Treatment of Manifestations
The American Academy of Pediatrics and American College of Medical Genetics and Genomics (ACMG) have published patient management guidelines for children with NF1 [Miller et al 2019], and the ACMG has also published management guidelines for affected adults [Stewart et al 2018]. Similar recommendations have been made by other experts [Ferner & Gutmann 2013, Dunning-Davies & Parker 2016].
Individuals with NF1 who have abnormalities involving the eye, central or peripheral nervous system, spine or long bones, cardiovascular system, or endocrine system should be referred to an appropriate specialist for treatment. Malignant tumors that develop in individuals with NF1 should be managed by surgical and/or medical oncologists who are familiar with the unusual molecular oncogenic mechanisms and inherent tumor predispositions (e.g., with radiotherapy [Evans et al 2002, Sharif et al 2006]; see Agents/Circumstances to Avoid) that may be associated with this genetic condition.
Neurofibromas. Discrete cutaneous or subcutaneous neurofibromas that are disfiguring or in inconvenient locations (e.g., at belt or collar lines) can be removed surgically, or, if small, by laser or electrocautery.
- Surgical removal of individual cutaneous neurofibromas is generally straightforward, but the large numbers of such tumors that occur in many affected adults may limit the extent of treatment that is practical or possible. Laser ablation is a rapid and effective method of removing larger numbers of cutaneous neurofibromas with satisfactory cosmetic results [Kriechbaumer et al 2014, Rosenbaum & Wimmer 2014, Méni et al 2015].
- Surgical treatment of plexiform neurofibromas is often unsatisfactory because of their intimate involvement with nerves and their tendency to grow back at the site of removal [Prada et al 2012, Nguyen et al 2013a, Rosenbaum & Wimmer 2014, Safaee et al 2015, Safaee et al 2017].
- In one small series in which surgical removal of superficial plexiform neurofibromas was undertaken in children while the tumors were still relatively small, it was possible to resect the neurofibromas without producing any neurologic deficit [Friedrich et al 2005].
- Recommendations for clinical management of orbital/periorbital plexiform neurofibromas in children with NF1 have been made by a multidisciplinary expert task force [Avery et al 2017].
- Clinical trials for several different medical treatments of plexiform and spinal neurofibromas are currently under way [Blakeley & Plotkin 2016, Karajannis & Ferner 2015] (see Therapies Under Investigation).
- Radiotherapy of plexiform neurofibromas is contraindicated because of the risk of inducing malignant peripheral nerve sheath tumors in these genetically predisposed individuals [Evans et al 2002].
Malignant peripheral nerve sheath tumors. Pain, development of a neurologic deficit, or enlargement of a preexisting plexiform neurofibroma may signal a malignant peripheral nerve sheath tumor and require immediate evaluation [Valeyrie-Allanore et al 2005]. Examination by MRI, PET, or PET/CT [Combemale et al 2014, Hirbe & Gutmann 2014, Salamon et al 2015, Van Der Gucht et al 2016] is useful in distinguishing benign and malignant peripheral nerve sheath tumors, but definitive differentiation can only be made by histologic examination of the tumor. Complete surgical excision, when possible, is the only treatment that offers the possibility of cure of malignant peripheral nerve sheath tumors [Dunn et al 2013, Valentin et al 2016]. Adjuvant chemotherapy or radiotherapy is sometimes used as well and appears to have benefitted some (but not most) patients with NF1 [Chaudhary & Borker 2012, Zehou et al 2013, Valentin et al 2016]. Clinical trials for malignant peripheral nerve sheath tumors are currently under way [Karajannis & Ferner 2015] (see Therapies Under Investigation).
Optic gliomas. Optic gliomas tend to occur at a younger age but to follow a more benign course in children with NF1 than in children who do not have NF1 [Nicolin et al 2009, Stokland et al 2010, Goodden et al 2014]. Most optic pathway gliomas found on MRI in people with NF1 are asymptomatic and do not require treatment [Blanchard et al 2016, Friedrich & Nuding 2016, Parkhurst & Abboy 2016, Sellmer et al 2018]. Chemotherapy is the treatment of choice for progressive optic pathway gliomas in children with NF1, although the results are mixed [Rosenfeld et al 2010, Ardern-Holmes & North 2011, Fisher et al 2012, Shofty et al 2015]. Children with NF1 and low-grade progressive gliomas (most of which were located in the optic pathways) had better survival after treatment with carboplatin and vincristine than children with similar tumors who did not have NF1 [Ater et al 2016]. Surgical treatment of optic nerve glioma is usually reserved for cosmetic palliation in a blind eye, and radiotherapy is usually avoided because of the risk of inducing malignancy or moya-moya in the exposed field [Evans et al 2002, Ullrich et al 2007a, Murphy et al 2015]. Clinical trials for optic pathway gliomas are currently under way [Karajannis & Ferner 2015] (see Therapies Under Investigation).
Brain tumors. Non-optic gliomas in people with NF1 tend to follow a less aggressive course than in those who do not have NF1 [Ullrich et al 2007a, Sellmer et al 2017]. Most such tumors are asymptomatic, and they usually grow slowly or not at all over many years [Sellmer et al 2017].
Orthopedic issues. Dystrophic scoliosis in children with NF1 often requires surgical management, which may be complex and difficult [Stoker et al 2012, Kawabata et al 2013, Deng et al 2017]. Nondystrophic scoliosis in persons with NF1 can be treated in a manner similar to idiopathic scoliosis.
Surgical treatment of tibial pseudarthrosis is difficult and often unsatisfactory [Stevenson et al 2013, Borzunov et al 2016].
Bisphosphonate treatment may benefit patients with NF1 who have osteoporosis, but the effect may be smaller than is usually seen in patients who do not have NF1 [Heervä et al 2014].
Neurobehavioral problems. Methylphenidate treatment often benefits children with attention-deficit/hyperactivity disorder and NF1 [Lion-François et al 2014].
Breast cancer. Although women with NF1 who develop breast cancer tend to have more aggressive disease than other women, the author is not aware of any evidence or recommendations that they should be treated differently from others with similar pathology and tumor markers. The author is not aware of any secondary tumors related to radiotherapy for breast cancer in women with NF1; however, avoiding radiotherapy, if possible, is reasonable.
Surveillance
The American Academy of Pediatrics and ACMG have published guidelines for surveillance of children with NF1 [Miller et al 2019], and the ACMG has published similar guidelines for affected adults [Stewart et al 2018]. Recommendations regarding surveillance of NF1 patients have also been made by other experts [Ferner & Gutmann 2013, Dunning-Davies & Parker 2016].
The following are recommended:
- Annual physical examination by a physician who is familiar with the individual and with the disease
- Annual ophthalmologic examination in early childhood; less frequent examination in older children and adults
- Regular developmental assessment by screening questionnaire (in childhood)
- Regular blood pressure monitoring
- Other studies (e.g., MRI) only as indicated on the basis of clinically apparent signs or symptoms
- Monitoring of those who have abnormalities of the central nervous system, skeletal system, or cardiovascular system by an appropriate specialist
Because of the increased risk of developing breast cancer before age 50 years among women with NF1 [Madanikia et al 2012, Wang et al 2012, Seminog & Goldacre 2015], US National Comprehensive Cancer Network Guidelines recommend that mammography be performed annually beginning at age 30 and that breast MRI be considered between ages 30 and 50 years in women with NF1 [Daly et al 2017]. However, the efficacy and cost-effectiveness of such screening have not yet been demonstrated [Howell et al 2017].
Agents/Circumstances to Avoid
No limitations are necessary for most individuals with NF1. Limitations may be required if certain particular features such as tibial dysplasia or dysplastic scoliosis are present; in these instances the limitation is determined by the feature, not by the presence of NF1 itself.
Radiotherapy of individuals with NF1 appears to be associated with a high risk of developing malignant peripheral nerve sheath tumors within the field of treatment [Evans et al 2002, Sharif et al 2006].
Evaluation of Relatives at Risk
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Pregnancy Management
Although most pregnancies in women with NF1 are normal, serious complications can occur [Chetty et al 2011, Terry et al 2013].
- Many women with NF1 experience a rapid increase in the number and size of neurofibromas during pregnancy.
- Hypertension may first become symptomatic or, if preexisting, may be greatly exacerbated during pregnancy.
- Large pelvic or genital neurofibromas can complicate delivery, and cesarean section appears to be necessary more often in pregnant women with NF1 than in other women.
Therapies Under Investigation
Various medical treatments for plexiform and spinal neurofibromas are being evaluated in clinical trials [Blakeley & Plotkin 2016, Karajannis & Ferner 2015]. A 20% or greater decrease in tumor volume was observed in 17 of 24 children with inoperable symptomatic or health-threatening plexiform neurofibromas who received long-term treatment with selumetinib, a MEK inhibitor, in a Phase I clinical trial [Dombi et al 2016]. Tumor progression did not occur in any case, and toxicity was considered to be acceptable in this trial (see Note).
Radiofrequency therapy has shown promise for treatment of facial diffuse plexiform neurofibromas and café au lait spots in small clinical series [Baujat et al 2006, Yoshida et al 2007].
Controlled trials of several therapeutic approaches to malignant peripheral nerve sheath tumors are available to individuals with NF1 [Karajannis & Ferner 2015] (see Note).
Several controlled trials for treatment of optic pathway gliomas are available to individuals with NF1 [Karajannis & Ferner 2015] (see Note).
Clinical trials have been undertaken for the learning and behavioral problems that occur in people with NF1 [Lion-François et al 2014, van der Vaart et al 2016] (see Note).
Note: NIH ClinicalTrials.gov has a list of current clinical trials for NF1.
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for access to information on clinical studies for a wide range of diseases and conditions.
Other
Hormonal contraception appears not to stimulate the growth of neurofibromas in women with NF1 [Lammert et al 2006].