Myoclonic Epilepsy Associated With Ragged-Red Fibers
A number sign (#) is used with this entry because this syndrome represents a phenotype that can be produced by mutation in more than 1 mitochondrial gene, e.g., MTTK (590060), MTTL1 (590050), MTTH (590040), MTTS1 (590080), MTTS2 (590085), MTTF (590070). Features of the MERRF syndrome have also been associated with mutation in the MTND5 gene (516005).
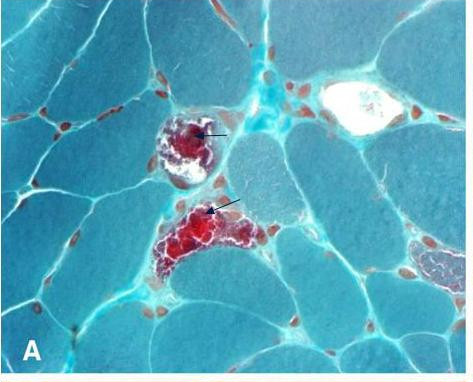
Clinical FeaturesFukuhara et al. (1980) provided an early report of myoclonic epilepsy associated with ragged-red fibers (MERRF). For detailed clinical features, see MOLECULAR GENETICS
InheritanceRosing et al. (1985) described an extensive family in which many members showed this combination of abnormalities which goes by the acronymic designation MERRF syndrome. Autosomal dominant, autosomal recessive, and X-linked inheritance could be excluded. Variability of expression and variable characteristics of the inheritance were consistent with mutation in mitochondrial DNA. The clinical spectrum was considered compatible with the proportionality model of mutant and wildtype mtDNAs. Serum levels of pyruvate or pyruvate and lactate were elevated.
Although the genetic defect is transmitted through the maternal lineage, the clinical phenotype varies greatly within a pedigree, consistent with a heteroplasmic population of mtDNAs, some of which are wildtype and others mutant. In skeletal muscle, the biochemical defect is often segmental (Matsuoka et al., 1991), suggesting a nonrandom distribution of mutant and wildtype mtDNAs within a muscle cell.
Molecular GeneticsA specific mutation in mitochondrial DNA was first demonstrated by Shoffner et al. (1990) (MTTK, 590060.0001). The A-to-G mutation at nucleotide 8344 accounts for 80 to 90% of MERRF cases (Shoffner and Wallace, 1992). Biochemically, the mutation produces multiple deficiencies in the enzyme complexes of the respiratory chain, most prominently involving NADH-CoQ reductase (complex I) in cytochrome c oxidase (COX) (complex IV), consistent with a defect in translation of all mtDNA-encoded genes (Wallace et al., 1988; Bindoff et al., 1991). Chomyn et al. (1991) showed that transfer of mtDNAs carrying the mutation to human cell lines lacking their own mitochondrial DNA resulted in a severe defect in mitochondrial translation in the recipient cells, independent of nuclear background, implying that the tRNA mutation itself is sufficient to cause the disease.
Holme et al. (1993) reported a woman with multiple symmetric lipomas (MSL; see 151800) in the neck and shoulder area associated with a heteroplasmic c.8344A-G mutation in the MTTK gene (590060.0001). Her son, who also carried the mutation, had MERRF syndrome; the mother had no signs of MERRF syndrome. The fraction of mutant mtDNA in the woman varied between 62% and 80% in cultured skin fibroblasts, lymphocytes, normal adipose tissue, and muscle, whereas the fraction of mutant mtDNA in the lipomas ranged from 90 to 94%. Ultrastructural examination of the lipomas revealed numerous mitochondria and electron-dense inclusions in some adipocytes. Holme et al. (1993) concluded that the mutation may either directly or indirectly perturb the maturation process of the adipocytes, increasing the risk of lipoma formation.
In several affected members of 3-generation Sardinian kindred with a maternally inherited syndrome characterized by features of both MERRF and MELAS (540000), Zeviani et al. (1993) identified a mutation in the MTTK gene (590060.0002). The relative amount of mutant mtDNA in muscle correlated with the severity of the clinical presentation. Clinical features included myoclonic epilepsy, neural deafness, ataxia, and stroke-like episodes.
In a mother and daughter with MERFF/MELAS overlap syndrome, Nakamura et al. (1995) identified a heteroplasmic mutation in the MTTS1 gene (590080.0001). The proband in their study was a mentally retarded 26-year-old woman who had had epileptic attacks since the age of 15 years. At the age of 20 years, clear symptoms of MERRF syndrome developed, including myoclonic seizures, generalized tonic-clonic seizures, and paroxysmal hearing disturbance. She also showed mental deterioration, muscle atrophy weakness, and truncal ataxia. Lactate levels in both blood and cerebrospinal fluid were elevated. The brain CT scan showed cerebral atrophy and bilateral calcification of the basal ganglia. Muscle biopsies showed many ragged-red fibers and abnormal mitochondria with concentric cristae. The mother was a 55-year-old woman who had myoclonic jerks of the arms and generalized seizures since the age of 37 years. At age 47 years, she was moderately demented. Muscle weakness and ataxia were not apparent. The brain CT scan revealed calcification of the basal ganglia and bilateral occipital lobe atrophy. At age 55 years, she developed blindness after an episode of generalized seizure, and thereafter was bedridden and severely demented; the phenotype suggested stroke-like episodes consistent with MELAS syndrome.
Melone et al. (2004) reported a 20-year-old man who experienced sudden migrainous headache and vomiting, followed by left hemiparesis and lateral homonymous hemianopia. Seizures also occurred. The clinical picture was consistent with MELAS syndrome. At age 25 years, he developed myoclonus and ataxia, suggesting progression to MERRF syndrome. His mother had shown similar stroke-like episodes and had died at age 36 years. Muscle biopsy of the proband showed abnormal mitochondrial proliferation and COX-negative fibers. Genetic analysis identified a heteroplasmic mutation in the MTTH gene (590040.0003).
Mancuso et al. (2004) reported an Italian woman with MERRF syndrome who experienced panic attacks at age 11 years. In her twenties, she developed migraine and progressive limb myoclonus. In her thirties, she had exercise intolerance, loss of balance, and memory problems, and later developed bilateral sensorineural hearing loss and mild cognitive deficits. Other features included short stature, pes cavus, ataxia, and mild ophthalmoparesis. Skeletal muscle biopsy showed multiple COX-negative fibers and ragged red fibers. Genetic analysis identified a heteroplasmic mutation in the MTTF gene (590070.0002).
Blakely et al. (2009) reported a woman who developed myoclonic jerks and generalized seizures at age 27 years, acute bilateral sensorineural hearing loss at age 37, underwent bilateral cataract surgery at age 39, and showed progressive loss of balance and arm weakness at age 47. Physical examination at age 49 showed some retinal pigmentary changes, dysarthria, proximal muscle weakness, and cerebellar ataxia. Skeletal muscle biopsy showed COX deficiency and ragged red fibers, consistent with mitochondrial accumulation. Genetic analysis identified a heteroplasmic mutation in the MTTP gene (590075.0003). The mutation segregated with cytochrome c oxidase activity in single muscle fibers.