Melanosis, Neurocutaneous
A number sign (#) is used with this entry because neurocutaneous melanosis (NCMS) is caused by somatic mutation in the NRAS gene (164790) on chromosome 1p13.
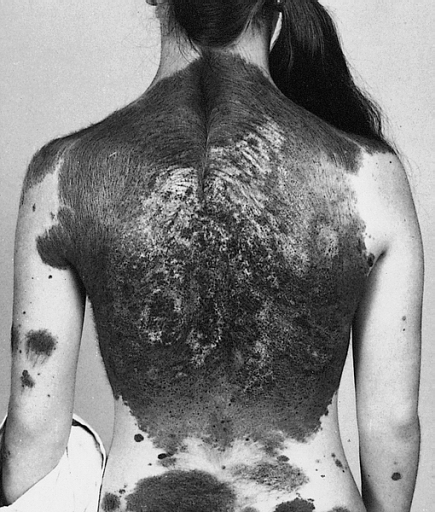
DescriptionNeurocutaneous melanosis, or neuromelanosis, is characterized by the presence of melanin-producing cells within the brain parenchyma or leptomeninges, which may lead to clinically apparent neurologic signs and symptoms, such as seizures. Other neurologic abnormalities, including hydrocephalus, arachnoid cysts, tumors, and syringomyelia, may also occur. The disorder is a rare but severe manifestation of congenital melanocytic nevus syndrome (CMNS; 137550). Some patients with neurocutaneous melanosis or CMNS may develop malignant melanoma. The incidence of neurologic involvement, development of malignant melanoma, and death is significantly associated with the projected adult size of the largest congenital melanocytic nevus, particularly those greater than 40 cm (summary by Kinsler et al., 2008; Kinsler et al., 2013).
Clinical FeaturesDemirci et al. (1995) described the MRI appearance of the brain in 2 cases of neurocutaneous melanosis. This demonstrated intraparenchymal melanin deposits but no detectable leptomeningeal abnormality.
Foster et al. (2001) reported follow-up of 46 patients with giant congenital melanocytic nevi (137550), including 42 who underwent magnetic resonance imaging. MRI abnormalities were detected in 14 of 42 children, most of whom were under 12 months of age. Ten (23%) of the 14 had central nervous system melanosis; less common findings included arachnoid cyst, Chiari type I malformation, and tethered spinal cord, in 1 patient each. Clinical follow-up for an average of 5 years found that only 1 of 46 patients developed neurologic symptoms, including developmental delay, hypotonia, and possible seizures; this patient had neuromelanosis. None of the 46 patients developed a cutaneous or central nervous system melanoma. Neither the location nor the size of the nevus appeared to correlate with the presence or absence of MRI abnormalities. Foster et al. (2001) concluded that central nervous system melanosis is not a rare event in patients with giant congenital melanocytic nevi, but that most patients with abnormal findings remain asymptomatic.
Kinsler et al. (2008) performed a retrospective study of 120 patients with congenital melanocytic nevi referred to a dermatology clinic between 1991 and 2007 and who underwent central nervous system imaging. None had neurologic complications at the time of referral. The mean age at first MRI was 2.46 years. MRI abnormalities were found in 22 (18%) of patients and were significantly associated with projected adult size of the CMN, particularly greater than 40 cm. The most common finding was neuromelanosis, which was found in 15 patients. Rare findings included choroid plexus papilloma, hydrocephalus, astrocytoma, and posterior fossa arachnoid cyst. Thirteen patients developed neurologic symptoms, including seizures, hemiparesis, reduced muscle tone, spasticity, ataxia, and/or global developmental delay. Five patients with MRI abnormalities remained asymptomatic at a mean age of 3.7 years. In addition, 5 patients with normal brain imaging showed neurologic symptoms. Two of the 120 patients developed fatal malignant melanoma and 1 died of aggressive and proliferative leptomeningeal melanosis, yielding an overall death rate of 2.5%. There was no significant association between CMN distribution and adverse outcomes.
Ramaswamy et al. (2012) retrospectively reviewed 14 cases of neurocutaneous melanocytosis. Six patients had died, including 2 in early childhood, 3 in childhood, and 1 at age 28 years. All who died had diffuse leptomeningeal deposits in both the brain and spine. Five developed hydrocephalus with rapidly increasing intracranial pressure; 4 had leptomeningeal melanoma. The most common site for melanocytic deposits was the temporal lobe. The living patients were between 1 and 7 years of age. Only 1 patient had normal brain imaging, but she had focal temporal epilepsy. Three patients with multifocal melanocytic deposits were asymptomatic, and 1 patient with deposits and a spinal arachnoid cyst was asymptomatic. One patient had multifocal melanocytic deposits, seizures, and severe developmental delay. Two additional patients with multifocal deposits and spinal cysts had seizures, including 1 with developmental delay. Ramaswamy et al. (2012) concluded that patients with neurocutaneous melanocytosis can have a wide range of intracranial and intraspinal abnormalities as well as highly variable clinical outcomes.
Kinsler et al. (2013) studied tissue samples from 15 unrelated patients with congenital melanocytic nevi who ranged in age from 2 to 23 years. Routine brain imaging in the first year of life showed 7 patients with parenchymal neuromelanosis, 1 with frontal lobe meningioma, and 5 with normal findings. However, 1 patient with initial normal findings later developed leptomeningeal melanocytosis, and another later developed hydrocephalus associated with a choroid plexus papilloma. Three patients had significant neurologic findings, such as developmental delay and seizures, although 2 had spinal cord compression and 2 had increased intracranial pressure.
InheritanceFerris et al. (1987) described a patient with neurocutaneous melanosis and suggested that the segmental distribution, sporadic nature, and invariable discordance in identical twins for the occurrence of giant hairy nevi is consistent with the hypothesis that the disease is a consequence of one or more somatic mutations which would result in prenatal lethality if they occurred in the germline cells.
Molecular GeneticsKinsler et al. (2013) identified somatic oncogenic missense mutations affecting codon 61 of the NRAS gene in affected cutaneous and neurologic tissues from 12 of 15 patients with congenital melanocytic nevus syndrome and/or neurocutaneous melanosis. Affected skin samples from 10 of 13 patients carried a somatic heterozygous mutation, including 8 with Q61K (164790.0008) and 2 with Q61R (164790.0002). The same codon 61 mutation was found in each of the anatomically separate melanocytic nevi from the same patient. In addition, all 11 neurologic samples from 5 patients from whom neurologic tissue was available were positive for a somatic Q61K mutation; this included both melanocytic and nonmelanocytic tissue, such as a choroid plexus papilloma and meningioma. In patients with both neurologic and skin samples available, the same mutation was present in both affected tissues. None of the patients carried an NRAS mutation in the blood. Pre- and post-malignant skin tissue was available from a patient with malignant melanoma, which showed a progression from heterozygosity to homozygosity for the Q61K mutation with the onset of malignancy. Mutations at codon 61 in the NRAS gene affect the guanosine triphosphate-binding site and result in constitutive activation of NRAS. Kinsler et al. (2013) concluded that multiple congenital melanocytic nevi and neuromelanosis, as well as nonmelanocytic CNS lesions, result from somatic mosaicism, and that the mutation probably occurs in a progenitor cell in the developing neural crest or neuroectoderm. The findings also suggested that these mutations may be lethal in the germline. Three of the original 15 patients with CMNS did not have NRAS mutations.