Wilson Disease
A number sign (#) is used with this entry because Wilson disease is caused by homozygous or compound heterozygous mutation in the ATP7B gene (606882) on chromosome 13q14.
DescriptionWilson disease is an autosomal recessive disorder characterized by dramatic build-up of intracellular hepatic copper with subsequent hepatic and neurologic abnormalities.
De Bie et al. (2007) provided a detailed review of the molecular pathogenesis of Wilson disease.
Clinical FeaturesIn Wilson disease, the basal ganglia and liver undergo changes that express themselves in neurologic manifestations and signs of cirrhosis, respectively. A disturbance in copper metabolism is somehow involved in the mechanism. Low ceruloplasmin (117700) is found in the serum. Shokeir and Shreffler (1969) advanced the hypothesis that ceruloplasmin functions in enzymatic transfer of copper to copper-containing enzymes such as cytochrome oxidase. Supporting the hypothesis was the finding of markedly reduced levels of activity of cytochrome oxidase in Wilson disease and moderate reductions in heterozygotes.
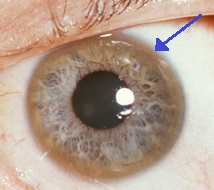
The Kayser-Fleischer ring is a deep copper-colored ring at the periphery of the cornea which is frequently found in Wilson disease and is thought to represent copper deposits. Bearn and McKusick (1958) and Whelton and Pope (1968) described azure lunulae of the fingernails in patients with Wilson disease. These are presumably of the same significance as the Kayser-Fleischer ring and possibly arise by the same mechanism. Hypercalciuria and nephrocalcinosis are not uncommon in patients with Wilson disease. Hypercalciuria associated with this disorder was first reported by Litin et al. (1959). Wiebers et al. (1979) observed renal stones in 7 of 54 patients with Wilson disease. Penicillamine therapy was accompanied by a decrease in urinary calcium excretion to normal values in 3 patients, but hypercalciuria persisted in 3. Azizi et al. (1989) described hypercalciuria and nephrolithiasis as presenting signs in Wilson disease and postulated tubular defect in calcium reabsorption. Hoppe et al. (1993) described a 17-year-old male with a 6-year history of hypercalciuria, nephrocalcinosis, and nephrolithiasis, in whom Wilson disease was finally diagnosed.
Bearn (1960) suggested that Jewish WND patients from eastern Europe are different from other groups of patients in that the age at onset is later, the disease is generally milder, and the serum copper and serum ceruloplasmin levels are 'particularly liable to be of normal concentration.' Bonne-Tamir et al. (1990) provided a full analysis of Wilson disease in Israel.
From a study of 28 Canadian families, Cox et al. (1972) suggested that there are at least 3 forms of Wilson disease. In a rare 'atypical form,' the heterozygotes show about 50% of the normal level of ceruloplasmin. This gene may have been of German-Mennonite derivation. In the 2 typical forms heterozygotes have normal ceruloplasmin levels, although they can be identified by decreased reappearance of radioactive copper into serum and ceruloplasmin. The authors referred to the 2 'typical forms' as the Slavic and the juvenile type. The Slavic type has a late age of onset and is predominantly a neurologic disease. The juvenile type, which occurs in western Europeans and several other ethnic groups, has onset before age 16 years and is frequently a hepatic disease. Czaja et al. (1987) demonstrated reduced ceruloplasmin gene transcription in 4 patients with Wilson disease (44% of controls). Low levels of ceruloplasmin are a normal finding in the newborn (Shokeir, 1971).
In Israel, Passwell et al. (1977) observed that Arab patients show an earlier age of onset and more severe course than Jewish patients. Within families of both ethnic groups, age of onset and type of disease show a close correlation. Thus, the authors concluded that the interethnic differences may reflect different mutations.
Fitzgerald et al. (1975) described a 57-year-old man with liver disease that they concluded represented Wilson disease. Ross et al. (1985) described a patient who was found to have hepatosplenomegaly at age 51, developed hand tremor at 52, and was having difficulty with hand dexterity at 55. The diagnosis of Wilson disease was made at age 58 on the basis of urinary, serum, and hepatic copper studies and liver histology, and despite the absence of Kayser-Fleischer rings. Wilson disease is not generally considered in patients over 30 years of age who present with liver disease and without neurologic signs. Danks et al. (1990) reported 4 such cases: 2 men, aged 43 and 48, and 2 women, aged 44 and 58. The 58-year-old woman had been ill for only 1 week and died in 36 hours of acute hepatorenal failure. Her sister had died of cirrhosis and liver failure at age 28. Alcohol intake was minimal or completely avoided in all. None of the known hepatitis viruses could be identified and no autoantibodies were detected.
Kuan (1987) demonstrated manifestations of myocardial involvement in Wilson disease. The occurrence of chondrocalcinosis and osteoarthritis in Wilson disease may be due to copper accumulation similar to the arthropathy of hemochromatosis (HFE; 235200) (Menerey et al., 1988).
Starosta-Rubinstein et al. (1987) correlated clinical manifestations with the findings of magnetic resonance imaging (MRI) of the brain. Van Wassenaer-van Hall et al. (1995) also used cranial MRI to study WND patients. Although the most striking findings on their MRI scan were abnormalities of the basal ganglia in generalized cerebral atrophy, they also noted subtle white matter abnormalities in some WND patients, particularly at the dentatorubrothalamic, pontocerebellar, and corticospinal tracts.
From Slovenia, Ferlan-Marolt and Stepec (1999) reported a 24-year-old woman with fulminant Wilsonian hepatitis accompanied by hemolytic anemia and leading to death in a few weeks. Kayser-Fleischer rings were said to have been absent, and there were no neurologic abnormalities until the development of the flapping tremor of hepatic failure in the last days of life.
Gu et al. (2000) studied mitochondrial function and aconitase activity in Wilson disease liver tissue and compared the results with those in a series of healthy controls and patients without Wilson disease. There was evidence of severe mitochondrial dysfunction in the livers of patients with Wilson disease. Enzyme activities were decreased as follows: complex I by 62%, complex II+III by 52%, complex IV by 33%, and aconitase by 71%. These defects did not seem to be secondary to penicillamine use, cholestasis, or poor hepatocellular synthetic function. Gu et al. (2000) stated that the pattern of enzyme defects suggests that free radical formation and oxidative damage, probably mediated via mitochondrial copper accumulation, are important in Wilson disease pathogenesis, and that their results provide a rationale for a study of the use of antioxidants in Wilson disease.
Both Wilson disease and hemochromatosis (235200), characterized by excess hepatic deposition of iron and copper, respectively, produce oxidative stress and increase the risk of liver cancer. Because the frequency of p53 mutated alleles (191170) in nontumorous human tissue may be a biomarker of oxyradical damage and identify individuals at increased cancer risk, Hussain et al. (2000) determined the frequency of p53 mutated alleles in nontumorous liver tissue from WND and hemochromatosis patients. When compared with the liver samples from normal controls, higher frequencies of G:C to T:A transversions at codon 249, and C:G to A:T transversions and C:G to T:A transitions at codon 250 were found in liver tissue from WND cases, and a higher frequency of G:C to T:A transversions at codon 249 was also found in liver tissue from hemochromatosis cases. Sixty percent of WND and 28% of hemochromatosis cases also showed a higher expression of inducible nitric oxide synthase in the liver, which suggested nitric oxide as a source of increased oxidative stress. The results were consistent with the hypothesis that the generation of oxygen/nitrogen species and unsaturated aldehydes from iron and copper overload in hemochromatosis and WND causes mutation in the p53 tumor suppressor gene.
Hedera et al. (2002) reported a 13-year-old male with Wilson disease who exhibited leukoencephalopathy early in the disease course. MRI showed increased signal intensities in the basal ganglia and throughout the subcortical white matter in the frontal lobes, which later extended to the parietal and occipital lobes.
Takeshita et al. (2002) investigated 2 families with Wilson disease in which sibs showed different clinical phenotypes and different ages at onset. In the first family, the second and fourth male children demonstrated onset of the neurologic type of Wilson disease at 16 and 28 years of age, respectively, and the first female child developed the hepatic type at 38 years of age. In family 2, the second male child showed neurologic symptoms at 32 years of age and was diagnosed as having the hepatoneurologic type of Wilson disease; the 35-year-old first female child was found to have the hepatic type in familial screening. In both families, affected individuals were compound heterozygotes for mutations in the ATP7B gene. In the first family, the mutations were R778L (606882.0009) and R919G (606882.0014). In the second family, the mutations were 2511delA (606882.0015) and A874V (606882.0016).
Hlubocka et al. (2002) studied 42 patients with Wilson disease (19 men and 23 women, mean age 34 +/- 10 years) and 42 age- and sex-matched healthy volunteers. All subjects underwent complete echocardiographic examination; 24-hour Holter monitoring was performed in 23 Wilson disease patients. In comparison with healthy subjects, patients with Wilson disease had increased thickness of the interventricular septum and left ventricular (LV) posterior wall. While the 2 groups did not differ in LV mass index, relative LV wall thickness was significantly increased in the Wilson disease patients compared to control subjects. Concentric LV remodeling was present in 9 patients (21%) and LV hypertrophy in 1 patient. Diastolic filling and the frequency of valvular abnormalities were comparable in both groups. Twenty-four-hour Holter monitoring detected ECG abnormalities in 10 patients (42%), the most frequent findings being runs of supraventricular tachycardias and frequent supraventricular ectopic beats.
Jung et al. (2005) reported a 17-year-old Korean man with Wilson disease who presented with polyneuropathy at least 6 months before developing more typical symptoms. Initial symptoms included intermittent paresthesia and weakness in both hands and feet with normal sensory examination. Nerve conduction studies and sural nerve biopsy were consistent with a mixed demyelinating and axonal neuropathy. Treatment with penicillamine, zinc sulfate, and vitamin B6 resulted in clinical improvement.
DiagnosisChowrimootoo et al. (1998) investigated the neonatal diagnosis of Wilson disease by measuring ceruloplasmin isoforms in neonatal cord blood samples and venous blood from both healthy adults and patients with Wilson disease. Total ceruloplasmin levels were reduced in all neonatal specimens. The plasma isoform, however, was significantly reduced or absent only in patients with Wilson disease, whereas the biliary isoform was reduced both in healthy neonates and patients with Wilson disease. The authors commented that measurement of ceruloplasmin isoforms in cord blood or dried blood spots may permit neonatal diagnosis of this condition, before substantial tissue damage has occurred.
Gow et al. (2000) reported their detailed experience of 30 patients with a diagnosis of Wilson disease seen in 2 Australian centers between 1971 and 1998. Twenty-two patients presented with chronic disease; age at diagnosis ranged from 7 to 58 years. Only 14 of these patients (64%) had Kayser-Fleischer rings; 5 of these had low serum ceruloplasmin concentrations and normal urinary copper excretion, 2 had normal ceruloplasmin levels and high urinary copper excretion, and 7 had the classic combination of low serum ceruloplasmin and high urinary copper. Eight patients presented with fulminant hepatic failure, with age at diagnosis ranging from 11 to 54 years; only 6 of these had Kayser-Fleischer rings, 7 had low serum ceruloplasmin, and 4 of them had raised urinary copper excretion. The others were anuric. Examination of the livers of these 8 patients, either at autopsy or posttransplantation, showed cirrhosis and elevated copper content. Gow et al. (2000) commented that the diagnosis of Wilson disease depended on the evaluation of clinical and laboratory evidence of abnormal copper metabolism, but that no single feature was reliable in isolation. Further, the authors suggested that Wilson disease should be considered in any patient at any age presenting with unusual liver or neurologic abnormalities.
Firneisz et al. (2001) described postmortem (postcremation) diagnosis of Wilson disease on the basis of skin cells left on the deceased's electric shaver. Foye (2001) and Kuruvilla (2001) took these authors to task, noting that the man's DNA added no new information since the same mutation was identified in the man's father and 2 children. Foye (2001) commented that with the growing array of available tests, 'we must always remember in each individual case to stop first and ask not just whether a particular test could be done, but whether it should be done.' Kuruvilla (2001) noted that the man had movement disorder for at least 10 years before his death and presented to his physician with parkinsonian symptoms and florid manifestations of cirrhosis. Because Kayser-Fleischer ring is present in 100% of patients with CNS manifestations of Wilson disease, neuroophthalmologic slit-lamp assessment is mandatory and cost effective in all patients suspected of having this disease.
Ferenci (2006) reviewed the geographic distribution of mutations in the ATP7B gene in Wilson disease patients to improve genetic diagnosis of Wilson disease. The most common mutation in patients from Europe is H1069Q (606882.0006). A unique 15-bp deletion in the 5-prime region (606882.0010) is frequent in Sardinia. M645R (606882.0020) is common in Spain, and R778L (606882.0009) is often found in patients from eastern Asia. Ferenci (2006) also presented a clinical algorithm for the diagnosis of Wilson disease.
Prenatal Diagnosis
Cossu et al. (1992) demonstrated how one can use flanking markers to do prenatal diagnosis by the linkage principle in this disorder. The probability of the fetus being affected was estimated to be only 0.007 in the example given.
Clinical ManagementSokol et al. (1985) successfully treated a 13-year-old girl with fulminant Wilson disease with orthotopic liver transplant. Polson et al. (1987) reported dramatic improvement in neurologic function over a period of 3 or 4 months after orthotopic liver transplantation. However, Guarino et al. (1995) published a case of a man treated with orthotopic liver transplantation who developed postoperative central pontine and extrapontine myelinolysis and then went on to develop new extrapyramidal symptoms 19 months after the liver transplant.
Lingam et al. (1987) showed that neurologic abnormalities can be reversed to some extent in children with Wilson disease. In some patients it was necessary to substitute triethylene tetramine (TETA) for penicillamine because of adverse effects of the latter agent. Wilson disease is effectively treated by any 1 of 3 drugs, D-penicillamine, trien, or zinc acetate (Brewer et al., 1987). Brewer et al. (1994) described the successful treatment with zinc acetate of 13 presymptomatic patients identified through screening of sibs. The levels of hepatic copper in response to several years of zinc therapy may remain the same, go down, or go up temporarily. This is a reflection of zinc induction of hepatic metallothionein, which sequesters copper in a nontoxic pool. Hepatic copper levels should not be used to manage therapy. Liver function is well preserved by zinc therapy, and Brewer et al. (1994) observed no zinc toxicity in these 13 patients. Brewer et al. (1994) reported that no patient developed symptoms related to Wilson disease. However, Lang et al. (1993) reported a 30-year-old patient who deteriorated at the end of the first month of zinc therapy and died in hepatic coma. Hoogenraad (1994) expressed doubt that zinc played a causal role in the worsening condition of the patient reported by Lang et al. (1993).
Devesa et al. (1995) described an uneventful pregnancy with delivery of a healthy newborn in a woman with Wilson disease who had been on the oral copper-chelating agent trientine (triethylenetetramine dihydrochloride) because of the development of nephrosis when D-penicillamine was used. Hartard and Kunze (1994) reported a successful pregnancy in a patient with Wilson disease treated with D-penicillamine and zinc sulfate 3 years prior to and during the pregnancy.
Brewer et al. (1998) presented data on the long-term follow-up of maintenance zinc treatment of 141 symptomatic and presymptomatic patients with Wilson disease. From these data, they concluded that zinc is effective as a sole therapy and that it has low toxicity. The authors also presented limited data on zinc treatment of children and pregnant women with Wilson disease which were also suggestive of efficacy and low toxicity.
LeWitt (1999) observed that 'Whereas management of Wilson's disease follows some of the most logical treatment strategies in all of clinical neurology, the optimal means for removing copper from the brain (and elsewhere) have not achieved consensus.' Articles by Walshe (1999), who defended the use of penicillamine, and by Brewer (1999) indicated that the role of penicillamine, now in its fifth decade of use, is still a matter of great controversy. Brewer (1999) suggested that penicillamine should not be used as initial therapy in Wilson disease. He cited a number of instances of penicillamine-induced worsening. He favored the use of zinc acetate for maintenance therapy of Wilson disease and mentioned other alternative therapies.
By genetic analysis, Wu et al. (2003) identified 17 presymptomatic patients with Wilson disease. Prophylactic treatment of 14 patients with zinc over 3 to 5 years resulted in decreased levels of urinary copper, which indicated effective control of copper metabolism. None of the patients developed clinical symptoms of Wilson disease or adverse effects of zinc therapy by the end of the study period. In contrast, 3 patients who refused treatment had symptomatic progression of the disease. Wu et al. (2003) concluded that presymptomatic DNA diagnosis of individuals at risk and zinc therapy are effective treatment.
Treatment for patients with Wilson disease who present with neurologic manifestations is difficult because penicillamine often makes them neurologically worse and zinc is slow acting. Brewer et al. (2003) performed an open-label study of 55 untreated patients presenting with neurologic Wilson disease and treated them with tetrathiomolybdate varying from 120 to 410 mg/day for 8 weeks and then followed up for 3 years. Only 2 patients treated with tetrathiomolybdate (4%) showed neurologic deterioration, compared with an estimated 50% of penicillamine-treated patients. Five of the 22 new patients exhibited bone marrow suppression and 3 had aminotransferase elevations. These numbers were higher than in the original 33 patients and appeared to be due primarily to a more rapid dose escalation. Brewer et al. (2003) concluded that tetrathiomolybdate shows excellent efficacy in patients with Wilson disease who present with neurologic manifestations. With rapid escalation of dose, adverse effects from bone marrow suppression or aminotransferase elevations can occur.
In a randomized controlled double-blind study of 48 patients with neurologic presentation of Wilson disease, Brewer et al. (2006) concluded that tetrathiomolybdate was a better choice than trientine for preserving neurologic function. Six (26%) of 23 patients treated with trientine showed neurologic deterioration during the 8-week study compared to 1 (4%) of 25 patients treated with tetrathiomolybdate.
Alvarez et al. (2010) described how tetrathiomolybdate (TM) inhibits proteins that regulate copper physiology. Crystallographic results revealed that the surprising stability of the drug complex with the metallochaperone Atx1 (602270) arises from formation of a sulfur-bridged copper-molybdenum cluster reminiscent of those found in molybdenum and iron sulfur proteins. Spectroscopic studies indicated that this cluster is stable in solution and corresponds to physiologic clusters isolated from TM-treated Wilson disease animal models. Finally, mechanistic studies showed that the drug-metallochaperone inhibits metal transfer functions between copper-trafficking proteins. Alvarez et al. (2010) concluded that their results are consistent with a model wherein TM can directly and reversibly downregulate copper delivery to secreted metalloenzymes.
MappingIn a large inbred kindred with affected persons in 2 generations, Frydman et al. (1985) investigated linkage of WND with 27 autosomal markers. A lod score of 3.21 was found at theta = 0.06 for linkage of WND and esterase D on chromosome 13. In a note added in proof, they indicated that they had typed a second unrelated 10-member sibship with WND; the maximum lod score was 1.48 at theta = 0, giving a combined maximum lod score of 4.55 at theta = 0.04. Bonne-Tamir et al. (1985, 1986) corroborated the linkage of WND and esterase D by studies of another inbred group, 2 unrelated Druze kindreds. The combined lod score was 5.49 at theta = 0.03. Bonne-Tamir et al. (1986) confirmed the localization of Wilson disease by demonstration of linkage to DNA markers on chromosome 13; their studies indicated that the WND locus is distal to the ESD locus. Yuzbasiyan-Gurkan et al. (1988) confirmed the linkage to markers on chromosome 13, with a maximum lod score of 2.189 at theta = 0.06 for linkage of Wilson disease to D13S1. One very informative pedigree was Hispanic. One pedigree in which affected persons had normal or low normal levels of serum ceruloplasmin (a finding in only about 15% of WND patients) showed a negative lod score. The proband was not on oral contraceptives, and there was no known ceruloplasmin-inducing factor present. The family was of Russian-Jewish background. By genetic linkage studies, Bowcock et al. (1988) narrowed the assignment of the WND locus to 13q14-q21. Farrer et al. (1988) explored the use of linked genetic markers to identify carriers, normals, and presymptomatic affected persons. A significant decrease on the average was found in serum copper concentrations in heterozygotes, but other sources of variation in serum copper concentration were much greater and precluded use of serum copper for carrier detection. A familial component, independent of WND genotype, appeared to be a major factor accounting for variation in ceruloplasmin levels among unaffected persons. Figus et al. (1989) found no recombination with ESD and found linkage to several RFLPs. With ESD and 1 closely linked RFLP, they could either define the carrier status or exclude homozygosity in most unaffected sibs. The linkage of the WND locus to ESD at 13q14 was first shown by studies using the isozymic polymorphism of esterase D in families of Middle Eastern origin. Using RFLPs detected by the ESD cDNA, Houwen et al. (1990) could not confirm this reported close linkage in an analysis of 17 families of northwestern European origin. However, no crossovers were detected in 63 meioses informative for linkage with marker D13S12, located more distally at 13q21. The data confirmed the assignment of WND to 13q14-q21. Its localization, however, seemed to be more distal to ESD than previously reported. In a study of 20 families, Scheffer et al. (1992) found that D13S31 was the closest proximal marker and D13S55 and D13S26 the closest distal markers. They identified a crossover between WND and D13S31 in 1 family and a crossover between WND and D13S55 in another. These crossover sites could be used as reference points for new chromosome 13q14-q21 markers for a more accurate mapping of the WND locus. Using D13S31 and D13S59 (the closest proximal and distal markers, respectively, for the WND locus) in fluorescence in situ hybridization studies of chromosomal aberrations, Kooy et al. (1993) determined that the Wilson disease locus is located at the junction of bands q14.3 and q21.1. In 51 families with Wilson disease, Thomas et al. (1994) studied DNA haplotypes of CA dinucleotide repeat polymorphisms in the 13q14.3 region. They found that 3 markers (D13S314, D13S133, and D13S316) showed nonrandom distribution on chromosomes carrying the WND mutation. They also found that haplotypes of these 3 markers had highly significant differences between WND and normal haplotypes in northern European families.
Molecular GeneticsBull et al. (1993) identified 2 patients with Wilson disease who were homozygous for a 7-bp deletion within the coding region of the ATP7B gene (606882.0001). Tanzi et al. (1993) identified 4 mutations in the ATP7B gene in unrelated persons with Wilson disease: 2 missense mutations (606882.0002-606882.0003) and 2 frameshift mutations resulting in a truncated gene product (606882.0004-606882.0005). The mutations were found among 50 unrelated families derived predominantly from the United States, 18 unrelated families from Russia, and 5 presumably unrelated families from Sicily. Clearly, Bull et al. (1993) and Tanzi et al. (1993) had independently isolated the same gene which was convincingly the one mutant in Wilson disease.
Thomas et al. (1995) reviewed the mutations found in the ATP7B gene. Their findings suggest a wide span in the age of onset of Wilson disease, perhaps wider than previously considered typical. Mutations that completely disrupt the gene can produce liver disease in early childhood at a time when Wilson disease may not be considered in the differential diagnosis.
Petrukhin et al. (1993) identified YACs spanning the Wilson disease region and derived cosmid contigs therefrom. Thirteen microsatellite markers were generated from cosmids and used for study of genetic equilibrium (linkage disequilibrium; LD). Strong LD was detected between these markers and the WND locus in 28 families from rural Russia, 43 families from Sardinia, and 67 families of predominantly North American and European descent. From their haplotype and mutation analyses, Petrukhin et al. (1993) predicted that approximately half of all Wilson disease mutations will be rare in the American and Russian populations.
Given the difficulties of searching for mutations in a gene spanning more than 80 kb of genomic DNA, haplotype data are important as a guide to mutation detection. Thomas et al. (1995) did haplotyping of the Wilson disease gene region in 58 families. These haplotypes, combining 3 markers (D13S314, D13S316, and D13S301), were usually specific for each different mutation. The haplotype data suggested that as many as 20 mutations might still be unidentified; a total of 25 disease-causing mutations had been identified at that time.
Genotype/Phenotype CorrelationsGromadzka et al. (2005) studied 142 Polish patients with Wilson disease and identified 26 mutations in the ATP7B gene: 11 truncating, 14 missense, and 1 splice site mutation. Patients with 1 or 2 truncating mutations on their alleles had lower serum copper and ceruloplasmin levels and were younger when the first symptoms of the disease appeared compared with individuals with 2 missense mutations, and the effect of truncating mutations on phenotype was dose-dependent. Gromadzka et al. (2005) found no association between type of ATP7B mutation and mode of initial disease presentation (neurologic, hepatic, or mixed).
Population GeneticsWhereas the worldwide prevalence of Wilson disease is estimated to be on the order of 30 per 1 million, with a gene frequency of 0.56% and a carrier frequency of 1 in 90, a higher prevalence seems to exist in Sardinia, where approximately 10-12 new cases per year are identified. Figus et al. (1995) analyzed mutations and defined the chromosomal haplotype in 127 patients of Mediterranean descent affected by Wilson disease: 39 Sardinians, 49 Italians, 33 Turks, and 6 Albanians. There were 5 common haplotypes in Sardinians, 3 in Italians, and 2 in Turks, which accounted for 85%, 32%, and 30% of the Wilson disease chromosomes, respectively. They identified 16 novel mutations: 8 frameshifts, 7 missense mutations, and 1 splicing defect. In addition, they detected 5 previously described mutations, e.g., his1070-to-gln (606882.0006), which accounted for 13% of the mutations in WND chromosomes in non-Sardinian Mediterranean populations.
In the Sardinian population, one haplotype accounts for 55% of WD chromosomes (Figus et al., 1995). Loudianos et al. (1999) characterized the putative promoter and 5-prime untranslated region of the WD gene and carried out mutation analysis in this region in Sardinian WD patients with the most common haplotype. They detected a single mutation resulting from a 15-nucleotide deletion (606882.0010) in all chromosomes with this common haplotype. With the addition of this mutation, the molecular defect has been found in 92% of the WD chromosomes in Sardinians.
Loudianos et al. (1998) performed a mutation screen on the WND gene in 59 patients of Mediterranean origin: 26 Continental Italians, 22 Sardinians, 9 Turkish, and 2 Albanians. They found 31 novel and 3 known mutations. Most of the patients were compound heterozygotes. Because there are so many causative mutations, the preclinical and prenatal diagnosis of Wilson disease should be carried out by a combination of mutation and linkage analysis.
Kim et al. (1998) identified 3 novel mutations in the ATP7B gene in Korean patients with Wilson disease. One of these, arg778 to leu (606882.0009), was found in 6 of 8 unrelated patients, giving an allele frequency of 37.5%.
Ha-Hao et al. (1998) performed mutation analysis in 33 German and 10 Cuban unrelated Wilson disease patients. The common his1069-to-gln (606882.0006) mutation accounted for 42% of all WND chromosomes in the German series and haplotype C was found to be highly predictive for this mutation. Six previously undescribed WND gene mutations were identified. In 15 German WND index patients and 3 sibs, both WND mutations could be determined and a genotype-phenotype correlation was attempted. Patients homozygous for the his1069-to-gln mutation showed almost a complete range of clinical presentations; thus, in this study, the his1069-to-gln mutation was not associated with a late neurologic presentation.
Okada et al. (2000) analyzed the ATP7B gene in 41 unrelated Japanese Wilson disease families, including 47 patients. They identified 21 mutations, 9 of which were novel.
Garcia-Villarreal et al. (2000) identified a founder mutation in the ATP7B gene (L708P; 606882.0023) in 18 individuals with Wilson disease from the Canary Islands of Spain. Twelve patients were homozygous for the mutation. Homozygous patients tended to have a neurologic presentation at an average age of 16 years. The L708P mutation was estimated to have arisen in Gran Canaria over 56 generations ago, in pre-Hispanic times. Garcia-Villarreal et al. (2000) estimated a prevalence for Wilson disease of 1 in 2,600 individuals in the Canary Islands.
Olivarez et al. (2001) undertook to estimate the frequency of Wilson disease in the U.S. Caucasian population. They used data from 4 studies to determine that approximately one-third of Wilson disease mutations in U.S. Caucasian Wilson disease patients are his1069-to-gln (606882.0006). They then determined the frequency of this mutation in random DNA samples from 2,601 U.S. Caucasian newborns to be 0.285%. Multiplying by 3 gave an estimated Wilson disease heterozygote frequency of 0.855% and an allele frequency of 0.428%, or 0.00428. These data gave a Wilson disease frequency of about 1 in 55,000 births. The 95% confidence interval was rather broad, ranging from about 1 in 18,000 to 1 in 700,000 births.
Margarit et al. (2005) analyzed 40 unrelated Spanish patients with Wilson disease and identified 21 different mutations in the ATP7B gene in 35 (87%) patients. The M645R (606882.0020) mutation was particularly prevalent and found in 22 patients (55%), who were all compound heterozygotes for mutation in the ATP7B gene. In 6 patients in whom M645R was combined with a nonsense mutation, there was early onset of the disease, occurring between 5 and 14 years of age.
Gupta et al. (2005) analyzed Indian patients with Wilson disease from 62 unrelated families and their first-degree relatives and identified a total of 9 mutations, 5 novel, in the ATP7B gene. The authors noted that homozygotes for different mutations that would be expected to produce similar defective proteins showed significant disparity in terms of organ involvement and severity of disease; in 1 family, 2 sibs with the same pair of mutant chromosomes had remarkably different phenotypes. Gupta et al. (2005) suggested that there may be as yet unidentified modifying loci that account for the observed phenotypic heterogeneity among patients with Wilson disease.
In 120 unrelated Korean patients with Wilson disease, Park et al. (2007) identified 28 different mutations, including 6 novel mutations, in the ATP7B gene. R778L (606882.0009) was the most common mutation, occurring in 39.2% of mutant alleles.
Mak et al. (2008) sequenced the ATP7B gene in 65 unrelated Han Chinese patients with Wilson disease and identified 126 disease alleles in 129 chromosomes (97.6% detection rate); the most prevalent mutation, R778L, was found in 22 chromosomes. The authors screened 660 healthy Hong Kong Han Chinese for R778L and a 2310C-G SNP in perfect linkage disequilibrium with R778L, and identified 3 carriers of both; neither variant was found in the remaining 657 individuals. Mak et al. (2008) calculated the prevalence of Wilson disease to be 1 in 5,400 in Hong Kong Han Chinese, and the East Asian-specific R778L mutation was estimated to have arisen 5,500 years earlier from a single ancestor.
In the Korean population, Park et al. (2009) found that the combined carrier frequency of 3 common ATP7B mutations, R778L, A874V (606882.0016), and N1270S (606882.0017), was 1 in 50 (2%). Extrapolating from this figure, the authors estimated that the carrier frequency of Wilson disease is about 1 in 27 in the Korean population, suggesting that the disorder is more common than in U.S. Caucasian populations.
Wang et al. (2011) identified 38 different pathogenic ATP7B mutations in 69 (69.86%) of 73 Chinese patients with Wilson disease. The most common mutation was R778L, which accounted for 23.29% mutant alleles, and the second most common mutation was I1148T (606882.0025), which accounted for 9.59% of mutant alleles.
Animal ModelLi et al. (1991) found biochemical and morphologic evidence to suggest that the Long-Evans Cinnamon (LEC) rat is an authentic model of Wilson disease. Canine copper toxicosis, an autosomal recessive disorder, is thought to be an authentic model of Wilson disease. Yuzbasiyan-Gurkan et al. (1993) found, however, that in the dog the disorder is not linked (within 13% recombination) to the RB1 locus (614041) or (within 5% recombination) to the ESD locus (133280). Furthermore, ESD and RB1, tightly linked in both the mouse and human genomes, were not found to be closely linked in the canine genome.
In the LEC rat, acute hepatitis develops spontaneously about 4 months after birth, with clinical features similar to those seen in human fulminant hepatitis, sometimes a feature of Wilson disease. Survivors of this often-fatal attack continue to suffer from chronic hepatitis and usually develop hepatocellular carcinoma at age 12 months or older. Copper is abnormally high in the liver of LEC rats, and hepatitis can be prevented by treatment with copper-chelating agents such as D-penicillamine. Wu et al. (1994) cloned cDNAs for the rat gene (Atp7b; 606882) homologous to the human Wilson disease gene and used them to identify a partial deletion in the gene in the LEC rat. The deletion removed at least 900 basepairs of the coding region at the 3-prime end, including the crucial ATP-binding domain, and extended downstream of the gene. The usefulness of the model for studying liver pathophysiology, for developing therapy for Wilson disease, and for studying the pathway of copper transport and its possible interaction with other heavy metals was noted.
Theophilos et al. (1996) cloned and sequenced the murine homolog of the WND gene (ATP7B). They demonstrated a point mutation in the 'toxic milk' (tx) mouse Wd gene. The coding sequence from the tx WND gene was identical to the sequence from the DL mouse except for a single base change (A4066G) in the mutant sequence. Theophilos et al. (1996) reported that this base change led to a met1356-to-val amino acid substitution within the proposed eighth transmembrane domain of the ATP7B protein. Theophilos et al. (1996) reviewed the pathophysiology of the disorder in the tx mouse. They noted that tx is an autosomal recessive mutation which leads to hepatic accumulation of copper from the third postnatal week. The pups are born with an apparent copper deficiency and the milk of the mutant mothers is deficient in copper, leading to continued copper deficiency in the pups. The authors noted that the pathology observed in the livers of the deficient mice shows significant differences from the liver pathology observed in Wilson disease. Huang and Gitschier (1997) pointed out that tx, in which the milk of mutant dams is fatally deficient in copper, has a parallel in 'lethal milk' (lm) in which the milk of mutant dams is fatally deficient in zinc. Copper deficiency in human milk in Wilson disease has, it seems, not been investigated. The gene that is mutant in 'lethal milk' of the mouse is zinc transporter-4 (602095).
The mouse homologs for the Menkes and Wilson disease genes are the mottled (Atp7a; 300011) and toxic milk (Atp7b) genes, respectively. These genes encode similar copper-transporting P-type ATPases. They are expressed in different adult tissues in patterns reflecting disease manifestations. Using RNA in situ hybridization, Kuo et al. (1997) determined the distribution of mottled and toxic milk transcripts during mouse embryonic development. The mottled gene was expressed in all tissues throughout embryogenesis and was particularly strong in the choroid plexuses of the brain. Contrary to the previous observation of absent or very low expression in adult liver, mottled was expressed in embryonic liver. Expression of the toxic milk gene was significantly more delimited, with early expression in the central nervous system, heart, and liver. Later in gestation, toxic milk transcript was clearly seen in liver, intestine, thymus, and respiratory epithelium, including nasopharynx, trachea, and bronchi. In lung, toxic milk expression was restricted to bronchi, while mottled expression was diffuse. Hepatic expression of both toxic milk and mottled was in the parenchyma, as opposed to blood cells. These results suggested that the mottled gene product functions primarily in the homeostatic maintenance of cell copper levels, while the toxic milk gene product may be specifically involved in the biosynthesis of distinct cuproproteins in different tissues.
Using homologous recombination to disrupt the normal translation of the Atp7b gene, Buiakova et al. (1999) generated a strain of mice that were homozygous null mutants for the Wilson disease gene. The Atp7b-null mice displayed a gradual accumulation of hepatic copper that increased to a level 60-fold greater than normal by 5 months of age. An increase in copper concentration was also observed in the kidney, brain, placenta, and lactating mammary glands of homozygous mutants, although milk from the mutant glands was copper deficient. Morphologic abnormalities resembling cirrhosis developed in most animals older than 7 months of age. Progeny of the homozygous mutant females developed neurologic abnormalities and growth retardation characteristic of copper deficiency. Copper concentrations in the livers of the newborn homozygous null mutants were decreased dramatically. Thus, the authors concluded that inactivation of the murine Atp7b gene produces a form of cirrhotic liver disease that resembles Wilson disease in humans and the 'toxic milk' phenotype in mice.
Terada et al. (1998) introduced human ATP7B cDNA into the LEC rat using recombinant adenovirus-mediated gene delivery. An immunofluorescence study and a subcellular fractionation study revealed the transgene expression in liver and its localization to the Golgi apparatus. Moreover, since the synthesis of holoceruloplasmin is disturbed in the LEC rat, the plasma level of holoceruloplasmin, oxidase-active and copper-bound form, was examined to evaluate the function of ATP7B protein with respect to copper transport. Holoceruloplasmin was found in plasma of LEC rats who received ATP7B cDNA. Terada et al. (1998) concluded that introduced ATP7B protein may function in the copper transport coupled with the synthesis of ceruloplasmin and that the Golgi apparatus is the likely site for ATP7B protein to manifest its function.
By investigating the common autosomal recessive copper toxicosis in Bedlington terriers, van de Sluis et al. (1999) identified a new locus involved in progressive liver disease. Whereas the ATP7B gene mapped to canine chromosome 22q11, CO4107, a microsatellite marker showing close linkage to copper toxicosis, mapped to canine chromosome 10q26. A transcribed sequence identified from a CO4107-containing BAC was found to be homologous to a gene expressed from human chromosome 2p16-p13, a region devoid of any positional candidate genes.
In mouse hepatocytes, Lang et al. (2007) demonstrated that Cu(2+) induced the secretion of activated acid sphingomyelinase (SMPD1; 607608) from leukocytes, leading to ceramide release in and phosphatidylserine exposure on erythrocytes, which are events prevented by inhibition of Smpd1. In LEC rats, deficiency in or pharmacologic inhibition of Smpd1 prevented Cu(2+)-induced hepatocyte apoptosis and protected the rats from acute hepatocyte death, liver failure, and early death. Patients with Wilson disease showed elevated plasma levels of SMPD1 and displayed a constitutive increase of ceramide- and phosphatidylserine-positive erythrocytes. Lang et al. (2007) concluded that Cu(2+) triggers hepatocyte apoptosis through activation of acid sphingomyelinase and release of ceramide, suggesting a previously unidentified mechanism for liver cirrhosis and anemia in Wilson disease.
HistoryA tribute to Dr. S. A. Kinnier Wilson and a comprehensive review of Wilson disease were published in 1988; see Marsden and Fahn (1988), Critchley (1988), Wilson (1988), and Walshe (1988).
Walshe (1996) provided a review of the historical background of the treatment of Wilson disease, beginning with BAL, which had practical problems, and continuing with the chelating agent EDTA, which proved disappointing, and ending up with penicillamine (Walshe, 1956). Almost overnight, Wilson disease became one of the few inherited metabolic disorders for which there was effective therapy. So successful did this prove that the fact that zinc salts could block copper from the gut and could be of therapeutic value passed virtually unnoticed. Hoogenraad and van den Hamer (1983) described its use. The third 'decoppering agent,' developed in the 1970s, was triethylene tetramine, as the dihydrochloride (Trientine). Walshe (1996) stated that a major and perhaps unexpected problem when initiating treatment is giving a 'reasonably accurate prognosis.' This may be related to the large number of different mutations and possible compound heterozygotes resulting in the varying clinical syndrome and different responses to treatment. He raised the question that initial