Exostoses, Multiple, Type I
A number sign (#) is used with this entry because multiple exostoses type I (EXT1) is caused by heterozygous mutation in the gene encoding exostosin-1 (EXT1; 608177) on chromosome 8q24.
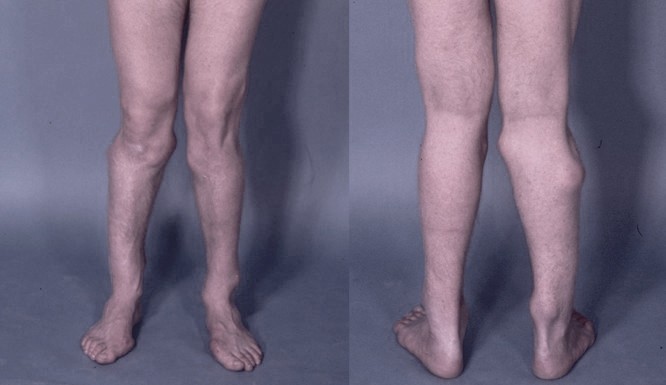
DescriptionMultiple hereditary exostoses (EXT) is an autosomal dominant disorder characterized by multiple projections of bone capped by cartilage, most numerous in the metaphyses of long bones, but also occurring on the diaphyses of long bones. Flat bones, vertebrae, and the ribs may also be affected, but the skull is usually not involved. Deformity of the legs, forearms (resembling Madelung deformity), and hands is frequent (Peterson, 1989).
Two conditions in which multiple exostoses occur are metachondromatosis (156250) and the Langer-Giedion syndrome (LGS; 150230); the latter condition is also known as trichorhinophalangeal syndrome type II. Furthermore, exostosis-like lesions occur with fibrodysplasia ossificans progressiva (FOP; 135100), occipital horn syndrome (304150), and the adult stage of hereditary hypophosphatemia (see 307800); these exostoses are located at sites of tendon and muscle attachment. A relatively rare variant of the supracondylar process, on the anteromedial surface of the distal humerus, can be confused with an exostosis; the variant is said to be present in about 1% of persons of European descent (Silverman, 1985).
Genetic Heterogeneity of Multiple Exostoses
Multiple exostoses type II (EXT2; 133701) is caused by mutation in the EXT2 gene (608210) on chromosome 11p11. Multiple exostoses type III (EXT3; 600209) has been mapped to a locus on chromosome 19.
Clinical FeaturesKrooth et al. (1961) reported on a study of the families of 6 persons with diaphyseal aclasis (multiple exostoses). The families were Chamorros, a Micronesian people who live in the Mariana Islands. The frequency of diaphyseal aclasis in the Chamorros of Guam was estimated at 1 in 1,000. In the 21 Guam cases, the tumors were evident on inspection in all males but in only half the females. In a study of 56 patients, Solomon (1963) found a sex ratio of 1 and reported that two-thirds of the patients had an affected parent.
Solomon (1964) observed 1 family in which all 8 affected persons in 4 sibships of 3 generations showed exostoses on the bones of the hands and fingers with very few elsewhere. In no other patients of his study did the abnormality take this particular form. Other workers had found no correlation between members of the same family as to form and distribution of disease. Solomon (1964) suggested that the family he reported may suffer from a rare disorder due to a gene distinct from that causing most cases.
Wicklund et al. (1995) conducted a retrospective review of 43 affected probands and 137 of their affected relatives. Penetrance appeared to be 100%. There was an excess of males within the entire affected population (104:76) and within identified probands (28:15). However, the male-to-female ratio was unskewed in nuclear families (probands, affected sibs, and parents). The excess of males appeared to be related to the fact that males have more severe and more frequent complications of EXT which do not have a primary genetic origin. Only 2.8% of the total affected population had experienced exostosis-related malignancy.
Quirini et al. (1996) described depression of the thoracic spinal cord in a 24-year-old male with a calcified osteochondroma contiguous with the upper endplate of T8. Del-Rio et al. (1992) described a 14-year-old boy in whom multiple exostoses had an unusual feature: exostoses on the distal phalanges were associated with malalignment and longitudinal dystrophy of the fingernails.
Reviewing a large cohort of 175 multiple exostoses patients referred to them over a 40-year period (1955 to 1995), Legeai-Mallet et al. (1997) found that 109 (62%) cases were familial and 66 (38%) were isolated. The disorder was consistently diagnosed before the age of 12 years, and the risk of malignancy, although increased, was modest (0.57%). The observation of 7 unaffected individuals (6 females, 1 male) with a family history and affected offspring supported the incomplete penetrance of the disorder. Moreover, the observation of an unequal sex ratio with a preponderance of males among the probands in this series (103 to 72, P less than 0.02) and in all reported series to date (198 to 133, P less than 0.001) gave support to the incomplete penetrance of EXT genes according to sex.
In 2 large consanguineous Pakistani families with typical features of multiple hereditary exostoses and mutations in EXT1 (see 608177.0010 and 608177.0011), Faiyaz-Ul-Haque et al. (2004) noted a previously unreported feature, bilateral overriding of single toes. No unaffected individuals had this feature, which was present at birth and allowed earlier diagnosis of the disorder.
Risk of Malignant Change
Voutsinas and Wynne-Davies (1983) suggested that the risk of malignant change in multiple exostoses is 0.5% (or 1.3% of those over 21 years). Matsuno et al. (1988) described a 19-year-old woman and a 29-year-old man with spindle-cell sarcoma as a complication of multiple exostoses. Both of the patients came from families with many affected members. Matsuno et al. (1988) stated that 20 to 50% of patients with multiple exostoses develop malignant change, as compared with 1 to 4% of persons with a solitary osteochondroma. That estimate of the frequency of malignant change may be abnormally high; the patients who get into trouble are those who come to medical attention.
In a review, Hennekam (1991) noted that malignancies probably occur in about 0.5 to 2% of cases. He quoted Ochsner (1978) who collected data on 59 patients with osteosarcoma in multiple exostoses. The mean age of onset of malignant degeneration was 31 years; it seldom occurred before the tenth or after the fiftieth year. The upper end of the femur and the pelvis were the main locations of exostoses, but they were also found in the shoulder girdle and the ribs.
Clinical ManagementPeterson (1989) discussed the orthopedic management of multiple hereditary exostoses.
MappingBuhler and Malik (1984) suggested that the mutation of multiple exostoses may be on 8q in the region of 8q24.1. They noted that the multiple exostoses of the Langer-Giedion syndrome are indistinguishable from those of the isolated disorder and that at least 1 case of trichorhinophalangeal syndrome without multiple exostoses had been found to have deletion in this region (Hamers et al., 1983). Buhler and Malik (1984) suggested that 2 closely linked loci may be situated at 8q24: 1 for type I trichorhinophalangeal syndrome (TRPS1; 190350) and 1 for multiple exostoses, and both may be deleted in LGS. Mental retardation present in some cases of LGS may be the result of deletion of additional neighboring genetic material.
Ogle et al. (1991) observed multiple exostoses in association with a balanced reciprocal translocation between 8q and 11p. The breakpoint on chromosome 8 was at proximal 8q24.1 within the critical region reported for Langer-Giedion syndrome.
Cook et al. (1993) found evidence for linkage of EXT to highly informative, short tandem repeat (STR) markers in the 8q24.11-q24.13 region, with genetic heterogeneity. A model of heterogeneity with linkage of the disease locus to the STR markers in 70% of the families (with a 95% confidence interval of 26 to 96%) produced a maximum lod score of 8.11, with the most likely position of the locus between D8S85 and D8S199.
Studying material from a patient with multiple exostoses and a translocation t(8;13) with a breakpoint at 8q23, Yoshiura et al. (1994) determined the order of 7 loci defined by cosmid clones by means of 2-color fluorescence in situ hybridization on elongated prophase chromosomes. They identified 2 flanking loci and by pulsed field gel electrophoresis with one of the flanking probes concluded that the probe lies less than 600 kb from the chromosomal breakpoint. Ludecke et al. (1995) and Hou et al. (1995) presented evidence that the Langer-Giedion syndrome is a true contiguous gene syndrome due to loss of functional copies of both the TRPS1 and the EXT1 gene and that the EXT1 gene is more than 1 Mb distal to the TRPS1 gene.
Blanton et al. (1996) studied 12 large multigenerational EXT families and found that the disorder mapped to 8q24 (EXT1) in 6 and to 11p (EXT2) in 6. None of the families mapped to the chromosome 19 locus (EXT3).
In 3 of 8 sporadic osteocartilaginous exostoses tumors, Mertens et al. (1994) found structural chromosomal rearrangements leading to loss of chromosome 8q24.1. The authors noted that multiple exostoses are part of the disease phenotype in patients with autosomal dominant LGS, many of whom have constitutional loss of genetic material from 8q24.1. Mertens et al. (1994) postulated the existence of a gene which carries a mutant allele that is inherited in the familial form of multiple exostoses and leads to the development of exostosis when a second mutation occurs as a somatic event in the other chromosome.
Pramparo et al. (2003) reported a family with multiple exostoses segregating with a reciprocal translocation, t(8;19)(q24.11;q13.13), in 8 members of 3 generations. FISH investigations detected a breakage of the dosage-sensitive EXT1 gene. Although 3 members of the family died perinatally from unknown causes and 1 carrier had 4 spontaneous abortions, the translocation was found only when a cytogenetic analysis was requested in an affected male because of oligozoospermia. Pramparo et al. (2003) noted that infertile males may be carriers of reciprocal or Robertsonian translocations with a higher frequency than the general population.
Locus Heterogeneity
Hall et al. (1985) could find no abnormality of 8q in cells cultured from an exostosis.
Using probe L48 at locus D8S51, Le Merrer et al. (1992) excluded the EXT gene from the 8q24.1 region. The cumulative lod score was -8.96 at theta = 0. In an addendum they stated that the testing of 3 additional families brought the lod score with probe L48 to -18.32 at theta = 0. In 2 Dutch EXT pedigrees with a total of 22 affected persons, Bakker et al. (1993) excluded linkage to the 8q22-q24 region, indicating genetic heterogeneity.
Legeai-Mallet et al. (1997) tested a series of 29 EXT families for possible linkage to the 3 known disease loci and estimated the probability of linkage of the disease to each locus by using an extension of the admixture test, which made modeling of heterogeneous monogenic disease feasible. A maximum likelihood was obtained for proportions of 44%, 28%, and 28% of families being linked to chromosomes 8, 11, and 19, respectively. No evidence of a fourth locus for the disease was found.
Francannet et al. (2001) reported a clinical and molecular study of 42 French families representing 217 affected individuals with multiple exostoses. Based on age of onset, number and location of exostoses, stature, and functional rating, they divided the affected individuals into those with a severe phenotype ('S') and those with a moderate phenotype ('M'). Seven of the 42 families belonged to group M, while the remaining 35 belonged to group S. Chondrosarcoma was found in 9 patients from 7 pedigrees, all of whom had type S. There was intrafamilial variability in severity of phenotype, and 4 families showed evidence of anticipation. The authors confirmed linkage to EXT1 in 29 of the families, to EXT2 in 9, and to EXT3 in 1. Francannet et al. (2001) concluded that there must be at least 1 additional locus for the multiple exostoses phenotype to account for the 3 unlinked families.
Molecular GeneticsIn 2 of 23 unrelated families with multiple exostoses type I, Ahn et al. (1995) identified a 1-bp deletion in the EXT1 gene (608177.0001) that segregated with the disease. In 4 of 6 EXT families demonstrating linkage to the EXT1 locus on chromosome 8, Hecht et al. (1997) identified 3 germline mutations in the EXT1 gene that segregated with the disease phenotype in each family (608177.0002-608177.0004). In 7 of 17 families (41%) with EXT, Philippe et al. (1997) identified mutations in the EXT1 gene, including 5 novel mutations (see, e.g., 608177.0007 and 608177.0009). Five of the families (29%) had mutations in the EXT2 gene.
Wuyts et al. (1998) analyzed the EXT1 and EXT2 genes in 26 EXT families originating from 9 countries. Of the 26 families, 10 had an EXT1 mutation and 10 had an EXT2 mutation. Twelve of these mutations had not previously been described. From a review of these and previously reported mutations, Wuyts et al. (1998) concluded that mutations in either the EXT1 or the EXT2 gene are responsible for most cases of multiple exostoses. Most of the mutations in these 2 genes cause premature termination of the EXT proteins, whereas missense mutations are rare. The development of exostoses is, therefore, mainly due to loss of function of EXT genes, consistent with the hypothesis that the EXT genes have a tumor suppressor function.
In 23 of 43 Japanese families examined, Seki et al. (2001) found 21 mutations, of which 18 were novel. Seventeen (40%) of the 23 families had a mutation in EXT1 and 6 (14%) had a mutation in EXT2. Of the 17 families with EXT1 mutations, 13 had those causing premature termination of the EXT1 protein, and 4 showed missense mutations. In contrast to the findings of Seki et al. (2001), Xu et al. (1999) detected more mutations in EXT2 than in EXT1 in Chinese patients (33% and 14%, respectively). An excess of EXT1 mutations was found in Caucasian patients, however, by Philippe et al. (1997) and Wuyts et al. (1998). In both Caucasian patients, as studied by Raskind et al. (1998), and Japanese patients, more EXT1 mutations were identified in familial cases than in sporadic cases.
In a study of 82 Japanese patients with hereditary multiple exostoses by Seki et al. (2001), 4 patients developed malignancy and their mutations (3 in EXT1 and 1 in EXT2) were all different, suggesting that malignant transformation is not directly related to a particular mutation in EXT1 or EXT2, but more likely involves other genetic factors. Loss of heterozygosity has been detected in chondrosarcoma not only at the EXT loci but also at others such as 10q (RET; 164761) and 3q.
Wuyts and Van Hul (2000) stated that to date, 49 different EXT1 and 25 different EXT2 mutations had been identified in patients with multiple exostoses, and that mutations in these 2 genes were responsible for over 70% of the EXT cases. The variety of mutations in each gene was detailed. Most of the mutations caused loss of function, which is consistent with the presumed tumor suppressor function of the EXT genes.
Genotype/Phenotype CorrelationsIn 36 of 38 EXT families linked to EXT1 or EXT2, Francannet et al. (2001) identified mutations: 27 were located in EXT1 and were almost randomly distributed over the 9 exons; 90% caused premature termination of the EXT1 protein. Nineteen of the EXT1 mutations were novel. No mutations were found in 2 EXT1-linked families. A more severe phenotype ('S') was shown to be significantly associated with EXT1 mutations, while a more moderate phenotype ('M') was associated with EXT2 mutations. One subgroup of the S phenotype, IS (10 to 25 exostoses, no vertebral exostoses, height below the 10th centile), was associated with mutations in EXT1 or EXT2. Mutations associated with another subgroup, IVS (very short stature), were located in exon 1 of EXT1. Chondrosarcomas were found only in patients with EXT1 mutations.
In 7 patients with EXT1 mutations and 16 patients with EXT2 mutations, Alvarez et al. (2006) analyzed the anatomic burden of disease by clinical and radiographic examination and evaluation of 76 phenotypic parameters. Patients with EXT1 mutation were found to have more exostoses, more limb malalignment with shorter limb segments and height, and more pelvic and flatbone involvement.
In 11 of 23 German patients with multiple exostoses, Heinritz et al. (2009) identified 11 different novel mutations in the EXT1 gene (see, e.g., 608177.0012). Eleven patients had mutations in the EXT2 gene, and 1 patient had no detectable mutations. Multiple splice site defects were identified. Although clinical details were limited, those with EXT1 mutations tended to have a more severe phenotype.
HistoryBeighton et al. (1993) analyzed the skeletons of adults in the Museum of Pathological Anatomy in Vienna. The museum was established in 1796 by Emperor Franz I, and is now housed in the Narrenturm, which is situated on the grounds of the Altes Allgemeines Krankenhaus, formerly a facility for the custodial care of persons with insanity. The museum contains 44,000 specimens. Beighton et al. (1993) pictured the skeleton of a man with multiple exostoses who died in 1842 of ruptured aortic aneurysm (probably syphilitic).