Pitt-Hopkins Syndrome
A number sign (#) is used with this entry because of evidence that Pitt-Hopkins syndrome (PTHS) is caused by heterozygous mutation in the TCF4 gene (602272) on chromosome 18q21.
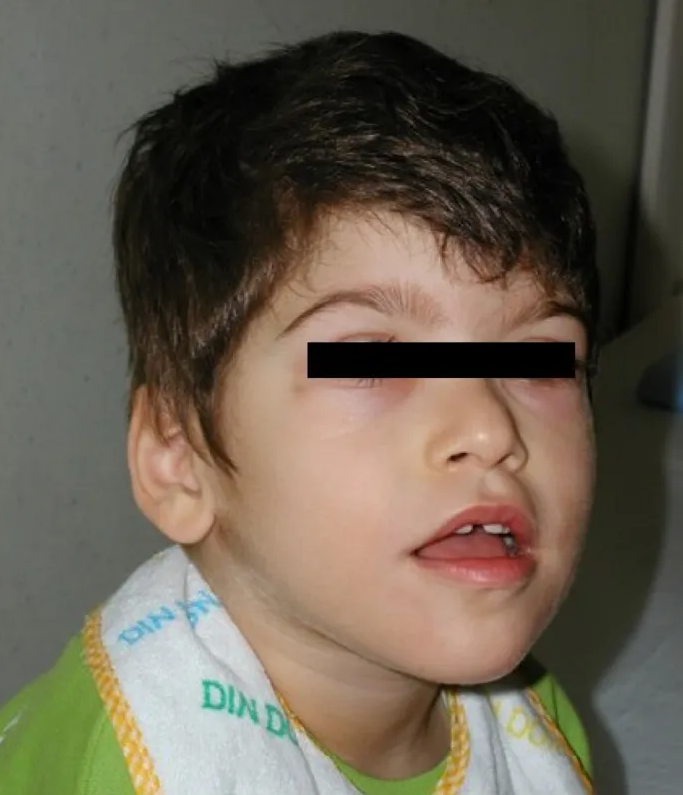
DescriptionThe Pitt-Hopkins syndrome is characterized by mental retardation, wide mouth and distinctive facial features, and intermittent hyperventilation followed by apnea (Zweier et al., 2007).
See also Pitt-Hopkins-like syndrome-1 (610042), caused by mutation in the CNTNAP2 gene (604569) on chromosome 7q35, and Pitt-Hopkins-like syndrome-2 (600565), caused by mutation in the NRXN1 gene (600565) on chromosome 2p16.3.
Clinical FeaturesPitt and Hopkins (1978) described 2 unrelated patients with a sporadic disorder comprising mental retardation, wide mouth, and intermittent overbreathing. The first patient, a male born of unrelated Greek parents, was profoundly retarded with poor muscular development. Head circumference and body measurements were normal. The mouth was wide with thick fleshy lips and a broad palate; the nose was beaked with broad nasal bridge, and the nostrils flared. There was bilateral pes cavus. Mild clubbing of the fingers and toes was present, and he had a left simian crease. An abnormal respiratory cycle was present every day, and showed extreme fluctuations. He overbreathed at up to 120 breaths per minute for 1 to 2 minutes and then had a period of apnea for up to 2 minutes, during the latter part of which he became cyanosed until a new episode of overbreathing terminated the cyanosis. The rhythm was absent at times during wakefulness and regularly during sleep, and increased with emotional stimuli. He died of pneumonia at the age of 20 years. The second patient, a female of unrelated Sicilian parents, was also retarded and had similar facial features. She was dwarfed and microcephalic, with everted feet and clubbing of fingers and toes. She also had an abnormal pattern of breathing with periodicity similar to that of the first patient. Electroencephalograms of both patients showed an excess of slow components.
Singh (1993) described a male patient with features similar to those of the patients reported by Pitt and Hopkins (1978), notably, wide mouth, thick lips, prominent nose, clubbing of fingers and toes, pes cavus, mental retardation, abnormal breathing pattern, and a history of epilepsy.
Van Balkom et al. (1998) described a similar female patient. Mental retardation and poor motor development were present. Daily episodic hyperbreathing, which caused massive swallowing of air and necessitated changing to clothes of a larger size during the daytime because of abdominal distention, was noted. Height and head circumference were below the 3rd centile. She had coarse hair, heavy eyebrows, a broad nasal bridge, large nose, flared nares, wide mouth with thick, fleshy lips, a broad palate, and an abnormal ear with a dysplastic helix on the right side. At the age of 40 years, all fingers as well as the great toes were clubbed.
Peippo et al. (2006) noted that to the time of their report, 4 patients with PTHS had been described. All showed dysmorphism consisting of large beaked nose, cup-shaped ears with broad helices, a wide mouth, cupid's-bow upper lip, wide and shallow palate, and broad or clubbed fingertips. They further defined the Pitt-Hopkins syndrome phenotype with a description of 2 new patients. In addition to severe developmental retardation, hypotonia, postnatal growth retardation, microcephaly, abnormal breathing, and characteristic dysmorphic features, both had epilepsy and intestinal problems with severe constipation in one and Hirschsprung disease (see 142623) in the other. Other abnormalities were hypopigmented skin macules in one and high-grade myopia in the other. Both had unusual frontal slow-and-sharp wave discharges on electroencephalography. MRI in both showed a similar hypoplastic corpus callosum with missing rostrum and posterior part of the splenium and bulbous caudate nuclei bulging towards the frontal horns. Amiel et al. (2007) ascertained 4 cases of PTHS. They noted that the abnormal ventilatory pattern characterized by daily bouts of diurnal hyperventilation that is the hallmark of PTHS was not reported in patients younger than 3 years of age. Epilepsy generally occurred later in the course of the disease.
Zweier et al. (2007) studied the 2 sporadic cases reported by Peippo et al. (2006) and 29 additional patients with severe mental retardation, breathing anomalies, and PTHS-like facial dysmorphism. These patients included the sib pair described by Orrico et al. (2001) and the patient of Van Balkom et al. (1998).
Brockschmidt et al. (2007) reported a girl with PTHS resulting from a 0.5-Mb microdeletion on chromosome 18q21.2. She had severely delayed psychomotor development, only achieving assisted walking at age 5 years. At age 7 years, she had no speech, hypotonia, and truncal ataxia. Dysmorphic features included coarse face with a broad and slightly depressed nasal bridge, wide mouth with a bow-shaped upper lip, short philtrum, dysplastic ears with anteverted earlobes, short neck, and low frontal and nuchal hairlines. Other features included widely spaced nipples, long tapering fingers, simian creases, proximally-inserted thumbs, and flat feet with superimposed toes. She had a happy disposition and began to have hyperventilation attacks at age 7.5 years.
Rosenfeld et al. (2009) identified 7 new cases of Pitt-Hopkins syndrome due to deletions of TCF4 and reviewed the 59 previously reported cases in the literature. Among their newly identified patients, all had features consistent with Pitt-Hopkins syndrome, although only 3 had breathing anomalies and none had seizures. Review of the literature indicated that although all reported patients had severe psychomotor retardation, the onset of seizures and hyperventilation episodes were limited to the first decade in most patients. Hyperventilation episodes were more common than seizures and were seen in the oldest patients, and individuals with missense TCF4 mutations were more likely to develop seizures.
Marangi et al. (2011) identified haploinsufficiency for the TCF4 gene in 14 of 63 Italian patients referred for suspicion of Pitt-Hopkins syndrome. One patient with the full syndrome had a balanced translocation involving the TCF4 gene. The patients ranged in age between 2 and 12 years, and all had severe intellectual disability with nearly absent language development. Eleven patients had a distinctive facial appearance, with bitemporal narrowing, square forehead, deep-set eyes, upslanted palpebral fissures, broad nasal bridge with pointed tip and flaring nostrils, full cheeks, protruding lower jaw and lip, and cup-shaped ears. Most (86%) had breathing abnormalities. Variable additional features included myopia, constipation, epilepsy, and uncoordinated movements. Marangi et al. (2011) noted the phenotypic overlap with Angelman (105830) and Rett (312750) syndromes, but concluded that the facial gestalt of PTHS combined with additional features can lead to the correct clinical diagnosis.
Lehalle et al. (2011) reported 4 unrelated patients with genetically confirmed PTHS who had fetal pads on the fingers and toes. They suggested that the presence of fetal pads can be a useful feature in the diagnosis of Pitt-Hopkins syndrome.
Clinical Variability
Zweier et al. (2007) failed to find a mutation in the TCF4 gene in 2 sibs described by Orrico et al. (2001), or in the patient reported by Van Balkom et al. (1998).
Kalscheuer et al. (2008) reported a girl with a de novo heterozygous balanced translocation t(18;20)(q21.1;q11.2) that disrupted the TCF4 gene and CHD6 gene on chromosome 20. She had mild to moderate mental retardation and minor facial anomalies, including a broad, square face, hypertelorism, flat nasal bridge, prominent ears, and a short neck. She also had mild hearing loss. However, she did not have features of the classic Pitt-Hopkins phenotype, such as breathing problems, hyperventilation, or epilepsy. PCR analysis showed that the breakpoints in TCF4 and CHD6 were in intron 3 and intron 1, respectively. Fusion transcripts were produced, with CHD6 exon 1 spliced to TCF4 exon 4. The findings indicated that not all mutations in TCF4 cause the severe PTHS phenotype.
DiagnosisWhalen et al. (2012) evaluated the clinical features of 112 patients with PTHS, 79 of whom had previously been reported, to better define the phenotype and allow for a more accurate clinical diagnosis. The most recognizable feature was the facial gestalt, including deep-set eyes, strabismus, myopia, marked nasal root, broad and/or beaked nasal bridge, large mouth, everted lower lip, tented upper lip, and/or prominent Cupid's bow, and ears with thick and overfolded helix. Of the 33 new patients, 63% had a single palmar crease, 65% had long, slender fingers, and 57% had flat feet. Intellectual disability was severe in all cases, and language was always absent or limited to only a few words. All had delayed walking, most had hypotonia (73%), and most had an ataxic or unsteady gait. Hyperventilation was present in over half of the patients, occurring spontaneously or triggered by emotional situations. Most (94%) also had stereotypic movements, particularly of the arms, wrists, and fingers. Most (89%) had a smiling appearance, as well as anxiety (81%). Variable features included constipation (77%) and cryptorchidism (33%). Less common features included microcephaly (7%), seizures (20%), and abnormalities on brain imaging (about 50%). Whalen et al. (2012) suggested and outlined a clinical diagnostic score for PTHS. The TCF4 mutational spectrum included 40% point mutations, 30% small deletions/insertions, and 30% deletions. Most of these were private mutations and generated premature stop codons. Almost all cases occurred de novo; 1 resulted from somatic mosaicism in the mother, and there was 1 pair of monozygotic twins. Missense mutations were localized in the bHLH domain, which is a mutational hotspot. There were no apparent genotype/phenotype correlations. The findings confirmed that TCF4 haploinsufficiency is the molecular mechanism underlying PTHS.
InheritanceIn all PTHS patients with heterozygous mutation in the TCF4 gene whose parents were available for analysis, the mutation was shown to occur de novo (Amiel et al., 2007; Zweier et al., 2007).
Molecular GeneticsBy array-comparative genomic hybridization in a patient with PTHS, Amiel et al. (2007) demonstrated a 1.8-Mb de novo microdeletion on 18q21.1; by molecular karyotyping with SNP arrays, Zweier et al. (2007) detected a 1.2-Mb deletion on 18q21.2 in another patient with this syndrome. In studies of patients with phenotypic features consistent with Pitt-Hopkins syndrome, both Amiel et al. (2007) and Zweier et al. (2007) demonstrated de novo heterozygous mutations in the TCF4 gene (see 602272.0001-602272.0004), which is located within the region of the deletion.
Brockschmidt et al. (2007) identified a de novo 0.5-Mb microdeletion of 18q21.2 encompassing the TCF4 gene in a girl with PTHS. RT-PCR analysis showed that the deletion resulted in functional TCF4 haploinsufficiency. The deletion occurred on the paternal chromosome.
Zweier et al. (2008) identified 16 different TCF4 mutations (see, e.g., 602272.0005-602272.0006) in 16 (14%) of 117 patients with a phenotype similar to PTHS. Thirteen of the mutations were frameshift, nonsense, or splice-site mutations, consistent with haploinsufficiency as the disease-causing mechanism.
De Pontual et al. (2009) identified 12 different mutations in the TCF4 gene among 13 patients with Pitt-Hopkins syndrome. A clustering of mutations in the basic domain of the E-protein indicated a mutation hotspot. In vitro studies demonstrated that wildtype TCF4 only activated the reporter construct when cotransfected with ASCL1 (100790) and ASCL1/TCF4 mutant heterodimers had decreased transcriptional activity compared to ASCL1/TCF4 wildtype heterodimers, consistent with a loss of TCF4 function. All mutations occurred de novo, except for 1 that was inherited from a mother who had chronic depression and epilepsy from age 20 years and was somatic mosaic for the mutation. In addition to severe mental retardation and characteristic facial features, all patients had low levels of IgM, but none showed features of an immunodeficiency. De Pontual et al. (2009) noted that the patients had been diagnosed over a 12-month period, suggesting that the disorder may be more common than originally thought.