Retinitis Pigmentosa 3
A number sign (#) is used with this entry because of evidence that retinitis pigmentosa-3 (RP3) is caused by mutation in the RPGR gene (312610) on chromosome Xp11.
Mutations in the RPGR gene can also cause X-linked cone-rod dystrophy (CORDX1; 304020) and a syndromic form of retinitis pigmentosa with deafness and sinorespiratory infections (300455).
DescriptionX-linked retinitis pigmentosa (XLRP) is a severe form of inherited retinal degeneration that primarily affects the rod photoreceptors (Demirci et al., 2002). It typically causes an early-onset night blindness and loss of peripheral vision, often causing patients to become legally blind by the age of 30 to 40 years. In RP3, affected males have a severe phenotype, and carrier females show a wide spectrum of clinical features ranging from completely asymptomatic to severe RP (Jin et al., 2007). Mutation in the RPGR gene is believed to account for approximately 70% of XLRP (Vervoort et al., 2000).
For a discussion of genetic heterogeneity of retinitis pigmentosa, see 268000.
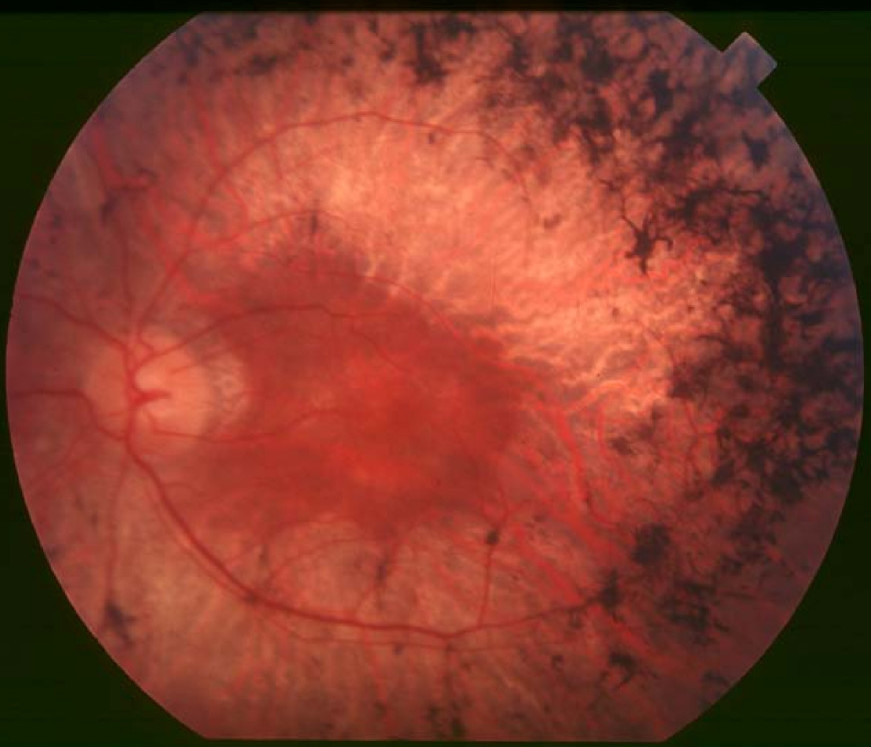
Clinical FeaturesFalls and Cotterman (1948) described an X-linked form of choroidoretinal degeneration which is distinguished from other types by the presence in heterozygous women of a tapetal-like retinal reflex (a brilliant, scintillating, golden-hued, patchy appearance most striking around the macula) but no visual defect.
Curtis and Blank (1989) studied a family in which a carrier female had an unusual tapetal reflex, the macula having 'a beaten metal appearance, with glistening patches.' The data supported the conclusion that retinitis pigmentosa with tapetal reflex is a separate entity.
Keith et al. (1991) described a large Australian family with extreme clinical variability in the hemizygotes: 1 member had typical rod-cone disease, 3 had the cone-rod pattern, and 1 had macroscopic changes in the macular area only, but showed low potentials in the ERG. From a study of reported case histories, Keith et al. (1991) concluded that clinical variability is a common feature of X-linked retinitis pigmentosa.
McGuire et al. (1995) studied 31 members of a 5-generation American family that included 7 affected females and 5 affected males. All 5 affected males showed diffuse retinal atrophy with round pigment cobblestone clumps, optic atrophy pallor with temporal loss, and myopia. Three young males when first tested at age 7 months and 11 years had nonrecordable electroretinograms and severely constricted visual fields. Six of the 7 affected females were evaluated several times over a period of 14 years. Five of these 6 had electroretinograms ranging from unrecordable to 80% of normal in the cone isolated ERG and ranging from nonrecordable to 45% of normal in the rod isolated ERG. Two affected females had moderately abnormal electroretinograms, which became nonrecordable and barely recordable after 14 years. In these 2 individuals, diffuse retinal atrophy was present with fine to granular pigment in equatorial regions. Mild nonprogressive myopia was present. McGuire et al. (1995) found that the clinical phenotype in the XLRP family studied by them was consistent with X-linked dominance, with expression milder and more variable in females.
Souied et al. (1997) described 9 families that showed an X-linked pattern of inheritance with a total of 28 affected males and 34 affected females. The females in these families met criteria for the diagnosis of retinitis pigmentosa. The males had a delayed onset of disease, with central vision being preserved until 40 to 45 years of age. Linkage to the RP3 locus was demonstrated, but SSCP and sequence analysis of the RPGR gene demonstrated no mutations. Four of the 9 families were later shown to have mutations at the RP3 locus (Rozet et al., 2002).
Grover et al. (2000) evaluated the progression of visual impairment in carriers of XLRP. They described the relationship between retinal findings at presentation and the extent of subsequent deterioration. They followed visual acuity, visual field, and electroretinograms in 27 carriers of XLRP and described 4 grades of fundus findings from grade 0 (normal) to grade 3 (diffuse changes). They found that carriers of XLRP with only a tapetal-like retinal reflex (grade 1) at presentation were more likely to retain visual function than those with peripheral retinal pigmentation. Grover et al. (2000) concluded that these data are useful in counseling such carriers about their visual prognosis.
Demirci et al. (2002) noted that affected males in the RP15 family studied by McGuire et al. (1995) and Mears et al. (2000) were reported to have early cone involvement, and the diagnosis of one of the patients from the series of Vervoort et al. (2000) was 'probable' X-linked cone dystrophy. Demirci et al. (2002) suggested that these patients may represent intermediate phenotypes within the broad spectrum of retinal disease caused by RPGR mutations.
Sandberg et al. (2007) measured the rates of visual acuity, visual field, and electroretinogram (ERG) loss in 2 large cohorts, one of patients with XLRP due to mutations in the RPGR gene (312610) and the other of patients with autosomal dominant RP due to mutations in the RHO gene (see 180380). Patients with RPGR mutations lost Snellen visual acuity at more than twice the mean rate of patients with RHO mutations. The median age of legal blindness was 32 years younger in patients with RPGR mutation than in patients with RHO mutations. Legal blindness was due primarily to loss of visual acuity in RPGR patients and to loss of visual field in RHO patients. Loss of acuity in RPGR patients appeared to be associated with foveal thinning.
Birch et al. (2015) compared the annual decline in visual field sensitivity in the transition zone at the edge of the frequency-domain optical coherence tomography (fdOCT) inner segment ellipsoid zone (EZ) to that at other locations in the visual field in 44 patients with RP3. Sensitivity just inside and outside the edge of the EZ declined at rates of 0.84 and 0.92 dB/year, respectively, whereas sensitivity in the macula and mid-periphery declined at rates of 0.38 and 0.61 dB/year, respectively. Birch et al. (2015) concluded that the edge of the EZ in patients with RP3 indicates a transition zone between relatively healthy and relatively degenerate retina.
In a study of 242 female carriers of X-linked RP, half of whom had RP2 or RP3, Comander et al. (2015) found that most carriers had mildly or moderately reduced visual function but rarely became legally blind. In most cases, obligate carriers could be identified by ERG testing. XLRP carrier ERG amplitudes and decay rates over time were on average half of those of affected men, consistent with the Lyon hypothesis of random X inactivation.
InheritanceJin et al. (2007) reported a 3-generation Japanese family with RP3 confirmed by genetic analysis (312610.0011). The proband's unaffected mother was an obligate carrier with somatic-gonadal mosaicism for the mutation. The mutation was identified in genomic DNA from ectodermal tissue, including hair follicles, hair shaft, and buccal cells, but not in mesodermal tissue such as lymphocytes. Jin et al. (2007) suggested that the mutation occurred during the mother's early embryonic development in a single progenitor cell from which both the gonads and ectodermal tissues were derived and that the mutation was subsequently passed on to her children and grandchild.
CytogeneticsFrancke et al. (1985) studied a male patient ('patient BB') with 3 X-linked disorders: chronic granulomatous disease with cytochrome b(-245) deficiency (CGD; 306400) and McLeod red cell phenotype (300842), Duchenne muscular dystrophy (DMD; 310200), and retinitis pigmentosa. A very subtle interstitial deletion of part of Xp21 was demonstrated. That this was a deletion and not a translocation was demonstrated by the absence of one DNA probe from the genome of the patient. The close clustering of CGD, DMD, and RP suggested by these findings was inconsistent with separate linkage data, which indicated that McLeod and CGD are close to Xg and that DMD and RP are as much as 15 cM from each other and far from Xg (perhaps at least 55 cM). At least 4 possible explanations of the discrepancy were proposed by Francke et al. (1985). One suggestion was that the deletion contained a single defect affecting perhaps a cell membrane component with the several disorders following thereon. Kunkel et al. (1985) mapped the deleted DNA fragment in this patient (see MAPPING).
MappingKunkel et al. (1985) developed a method for cloning the specific DNA fragment absent in patients homozygous or hemizygous for chromosomal deletions. They applied the method to the DNA of the patient with a minute interstitial deletion of Xp who was reported by Francke et al. (1985).
Ott et al. (1990) mapped the RP3 locus to a chromosome interval of less than 1,000 kb between the DXS1110 marker and the OTC locus (300461) at Xp21.1-p11.4.
Keith et al. (1991) found that the locus for RP in their large Australian family with extreme clinical variability in the hemizygotes was distal to L128 at Xp21.
Fujita et al. (1996) analyzed 27 individuals with X-linked RP from a large American family of apparent Irish descent, using 17 polymorphic markers for linkage analysis. Segregation of XLRP with markers in Xp21.1 was consistent with the RP3 subtype. A recombination proximal to DXS1110 (between markers DXS8349 and M6) was found in 1 patient with RP3, placing the mutation locus outside the deletion breakpoint, located about 40 kb centromeric to DXS11110, of patient BB reported by Francke et al. (1985).
McGuire et al. (1995) mapped a form of X-linked cone-rod degeneration, RP15, to the Xp22.13-p22.11 region. Mears et al. (2000) remapped the disorder in the family of McGuire et al. (1995) to a 19.5-cM interval on Xp21.1-p11.4 after clinical reevaluation of a female member. This new interval overlapped both the RP3 and the CORDX1 (304020) loci.
Molecular GeneticsMeindl et al. (1996) provided evidence that loss-of-function mutations within the RPGR gene (312610) are responsible for RP3 by identifying 2 small intragenic deletions and 2 nonsense and 3 missense mutations in highly conserved residues in unrelated patients with X-linked RP.
In an affected member of the family reported by McGuire et al. (1995), Mears et al. (2000) detected a de novo insertion in exon ORF15 of the RPGR gene (312610.0013); this exon had been identified by Vervoort et al. (2000), who found it to be a mutation hotspot. The identification of an RPGR mutation in a family with a severe form of cone-rod degeneration suggested that RPGR mutations may encompass a broader phenotypic spectrum than had previously been recognized in 'typical' retinitis pigmentosa.
In 4 of the 9 families with XLRP reported by Souied et al. (1997), Rozet et al. (2002) identified mutations in exon ORF15 of the RPGR gene. Rozet et al. (2002) also reported 5 additional affected families with mutations in ORF15. All 7 of the identified mutations were predicted to result in a truncated protein. Rozet et al. (2002) noted that the age at onset in affected females was delayed compared to affected males (20 to 40 years vs 10 to 20 years, respectively).
Demirci et al. (2006) reported a 16-year-old boy with RP and bilateral Coats-like vasculopathy (see 300216) in whom they identified a mutation in the RPGR gene (312610.0024). The mutation segregated with RP in the family, but clinical findings in other family members, including 2 affected male patients and 3 obligate carrier females, were consistent with typical X-linked recessive RP. Because the proband was the only family member who had Coats-like RP, Demirci et al. (2006) suggested that other genetic and/or environmental factors might be involved.
In affected individuals from an Israeli family with 'semi-dominant' X-linked retinitis pigmentosa, in which obligatory female carriers manifested high myopia, low visual acuity, constricted visual fields, and severely reduced electroretinogram amplitudes, Banin et al. (2007) identified a mutation in the RPGR gene (G275S; 312610.0003). The authors stated that obligate carriers from 2 unrelated Danish families in which Roepman et al. (1996) previously identified this mutation had no visual complaints and normal to slightly reduced retinal function. The disease-related RPGR haplotype of the Israeli family was found to be different from that of the 2 Danish families, indicating that the G275R mutation arose twice independently on different X-chromosome backgrounds. Genetic analysis excluded skewed X-inactivation patterns, chromosomal abnormalities, distorted RPGR expression levels, and mutations in 3 candidate genes as the cause for the differences in disease severity of female carriers. Banin et al. (2007) suggested that an additional gene or genes linked to RPGR modulate disease expression in severely affected carriers.
Branham et al. (2012) screened 214 male patients with simplex retinal degenerative disease, 185 with RP and 29 with cone/cone-rod dystrophy (COD/CORD), for mutations in the RPGR and RP2 genes. They identified pathogenic mutations in 32 (15%) of the patients. Four patients with COD/CORD had a mutation in the ORF15 mutation hotspot of the RPGR gene. Of the RP patients, 3 had mutations in RP2 and 25 had mutations in RPGR (including 23 in the ORF15 region). Branham et al. (2012) concluded that their results demonstrated a substantial contribution of RPGR mutations to retinal degenerations, and in particular to simplex RP. They suggested that RPGR should be considered as a first tier gene for screening isolated males with retinal degeneration.
Nishiguchi et al. (2013) identified a Japanese male patient with retinitis pigmentosa who was heterozygous for a frameshift mutation in the ciliary gene NEK2 (604043.0001), but who also carried a frameshift mutation in the known RP-associated RPGR gene (312610.0026) that had previously been described as a sufficient cause of X-linked RP by Vervoort et al. (2000); studies in zebrafish suggested that the RPGR allele interacts in trans with the NEK2 locus to exacerbate photoreceptor defects.
Genotype/Phenotype CorrelationsAmong female carriers from 45 families with RP3, Comander et al. (2015) found that those with RPGR ORF15 mutations tended to have worse visual function than those with RPGR exon 1 through 14 mutations.
Population GeneticsBuraczynska et al. (1997) stated that RP3 is the most frequent genetic subtype of X-linked retinitis pigmentosa.
Vervoort et al. (2000) found RPGR mutations in 72% of XLRP patients, of which 20% were in exons 1 through 14 and 80% were in the repetitive purine-rich sequence of exon ORF15. This suggested that at least 11% of all RP referrals may be accounted for by this locus.
Bader et al. (2003) screened 58 German XLRP families and found RP2 (300757) mutations in 8% and RPGR mutations in 71%, thus confirming the high frequency of the RP3 subtype.
NomenclatureInglehearn and Hardcastle (1996) took McGuire et al. (1995) to task for referring to the disorder as X-linked dominant. They pointed out that in the 1960s a consensus emerged that it was common in female carriers in all X-linked RP pedigrees to exhibit some symptoms on close examination and that high levels of intrafamilial variation in severity were common in heterozygotes. It was concluded at that time that there was no justification for categorizing X-linked RP into dominant, intermediate, and recessive forms and that one should simply speak of 'X-linked RP.' Inglehearn and Hardcastle (1996) pointed to the uncertainty of distinguishing the disorder in the family of McGuire et al. (1995) from RP6 (312612) on either clinical or genetic grounds.
In their reply to Inglehearn and Hardcastle (1996), Daiger et al. (1996) pointed out that 'the map is not the territory', i.e., names and symbols are only short-hand references to the actual phenotype or actual gene. 'To be more useful,' they continued, 'names should bear a reasonable relationship to the underlying entity, but names alone cannot substitute for actual clinical and genetic details.' With reference to the X-linked dominance of RP15 they stated that 'since all females with the proposed disease-causing gene are affected, the disease is 'dominant' in the traditional sense of the word,' but they agreed that the terms 'dominant' and 'recessive' can be misleading. They also noted that while it is possible that RP15 is actually RP6, the phenotypes of the 2 are distinct, and that 'cloning the gene or genes will settle this issue.'
HistoryIn a large kindred segregating for X-linked recessive retinitis pigmentosa with metallic-sheen fundus reflex in heterozygotes, Nussbaum et al. (1985) found measurable linkage to DXS7 (maximum lod = 2.5 at theta = 0.125). This is the same RFLP as that shown to be tightly linked to other forms of X-linked retinitis pigmentosa (XLRP) (Bhattacharya et al., 1984). The 95% probability limits are such that these findings might indicate allelism of these clinically different forms of RP. Studies with other RFLPs placed this form of RP distal to DXS7 on Xp.
Musarella et al. (1987) found close linkage of a form of X-linked RP and OTC (300461) with an anonymous DNA marker, 754, at Xp21 (interval = about 6 cM; lod = greater than 3.0). Chen et al. (1987) and Wirth et al. (1987, 1988) also found close linkage of one form of RP to OTC. Denton et al. (1988) did linkage studies in 3 large pedigrees segregating for the form of X-linked RP with the characteristic tapetal reflex in heterozygotes. Very close linkage to OTC was found (lod = 10.463 at theta = 0.01). Thus, the form of RP is probably that referred to here as RP3. It is also the locus presumably deleted in BB, the boy with RP, Duchenne muscular dystrophy, chronic granulomatous disease, and McLeod syndrome (Francke et al., 1985).
In another large kindred with X-linked retinitis pigmentosa and metallic sheen in the heterozygous carriers, Musarella et al. (1989) again found close linkage with Xp21 marker loci OTC and DXS206. By multipoint linkage analysis applying heterogeneity tests in 20 X-linked RP families, Musarella et al. (1990) concluded that the second X-linked RP locus may be located 8.5 cM proximal to DXS28 at Xp21.3. Chen et al. (1989) likewise found evidence of 2 distinct RP loci on Xp. In 1 family, they found the disease locus to be centromeric to DXS7, whereas in another family it was telomeric to DXS7. In 1 of 3 Swedish families, Dahl et al. (1991) demonstrated that the RP locus mapped to the same position as OTC and therefore represented RP3. In the other 2 families, linkage to OTC was excluded.